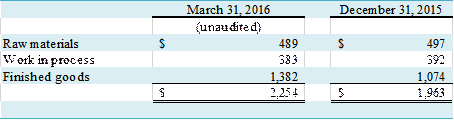UNITED STATES
SECURITIES AND
EXCHANGE COMMISSION
Washington, DC
20549
FORM 10-Q
[X] Quarterly
Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the quarterly period ended March 31, 2016
[ ] Transition Report Pursuant to
Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from _______________ to
______________
Commission File
Number: 0-18105
VASOMEDICAL, INC.
(Exact name of
registrant as specified in its charter)
Delaware 11-2871434
(State or other
jurisdiction of . (IRS
Employer Identification Number)
incorporation or organization)
137 Commercial
St., Suite 200, Plainview, New York 11803
(Address of
principal executive offices)
Registrant’s Telephone Number (516)
997-4600
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15 (d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was
required to file such reports), and (2) has been subject to such filing
requirements for the past 90 days. Yes
[X] No [ ]
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files). Yes
[X] No [ ]
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting company.
Large
Accelerated Filer [ ] Accelerated
Filer [ ] Non-Accelerated Filer
[ ] Smaller Reporting Company [X]
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act). Yes
[ ] No [X]
Number of Shares Outstanding of Common Stock, $.001 Par Value, at May 9, 2016 – 159,563,662
Vasomedical,
Inc. and Subsidiaries
INDEX
PART I – FINANCIAL
INFORMATION.. 3
ITEM 1 - FINANCIAL STATEMENTS. 3
CONDENSED
CONSOLIDATED BALANCE SHEETS as of March 31, 2016 (unaudited) and December 31,
2015. 3
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS (unaudited) for
the Three Months Ended March 31, 2016 and 2015. 4
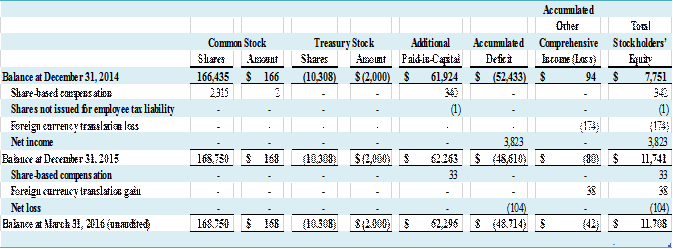
CONDENSED
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY for the Three Months Ended
March 31, 2016 (unaudited) and the Year Ended December 31, 2015. 5
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS (unaudited) for the Three Months Ended
March 31, 2016 and 2015. 6
NOTES
TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (unaudited) 7
ITEM 2 - MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS. 17
ITEM 4 - CONTROLS AND PROCEDURES. 22
PART
II - OTHER INFORMATION.. 23
ITEM 6 – EXHIBITS. 23
Vasomedical,
Inc. and Subsidiaries
(in thousands, except share and per share data)
The accompanying
notes are an integral part of these unaudited condensed consolidated financial
statements.
Vasomedical, Inc. and
Subsidiaries
(Unaudited)
(in thousands, except per
share data)
The
accompanying notes are an integral part of these unaudited condensed consolidated financial
statements.
Vasomedical, Inc. and
Subsidiaries
(in thousands)
The accompanying notes are an integral part of these unaudited
condensed consolidated financial statements.
Vasomedical, Inc. and
Subsidiaries
(Unaudited)
(in thousands)
The accompanying notes are an integral part of these unaudited
condensed consolidated financial statements.
NOTE A - ORGANIZATION AND PLAN
OF OPERATIONS
Vasomedical, Inc. was incorporated
in Delaware in July 1987. Unless
the context requires otherwise, all references to “we”, “our”, “us”, “Company”,
“registrant”, “Vasomedical” or “management” refer to Vasomedical, Inc. and its
subsidiaries.
Overview
Vasomedical, Inc. principally operates in three distinct
business segments in the healthcare equipment and information technology
industries. We manage and evaluate
our operations, and report our financial results, through these three business
segments.
·
IT segment, operating through a wholly-owned
subsidiary VasoTechnology, Inc., primarily focuses on healthcare IT and managed
network technology services;
·
Professional sales service segment, operating
through a wholly-owned subsidiary Vaso Diagnostics, Inc. d/b/a VasoHealthcare,
primarily focuses on the sale of healthcare capital equipment for large OEMs
into the health provider middle market; and
·
Equipment segment, operating through
wholly-owned subsidiaries Vasomedical Global Corp. and Vasomedical Solutions,
Inc., primarily focuses on the design, manufacture, sale and service of
proprietary medical devices.
VasoTechnology
VasoTechnology, Inc. was
formed in May 2015, at the time the Company acquired all
of the assets of NetWolves, LLC and its affiliates, including the membership
interests in NetWolves Network Services, LLC (collectively, “NetWolves”). It currently consists of a managed
network and security service division and a healthcare IT application VAR
(value added reseller) division.
Its current offerings include:
·
Managed diagnostic imaging applications
(national channel partner of GEHC IT).
·
Managed network infrastructure (routers,
switches and other core equipment).
·
Managed network transport (FCC licensed carrier
reselling 175+ facility partners).
·
Managed security services.
VasoTechnology uses a combination of proprietary technology,
methodology and third-party applications to deliver its value proposition.
VasoHealthcare
VasoHealthcare commenced operations in 2010, in conjunction
with the Company’s execution of its exclusive sales representation agreement
with General Electric Healthcare (“GEHC”), which is the healthcare business
division of the General Electric Company (“GE”), to exploit the sale of certain
healthcare capital equipment in the health provider middle market. Sales of GEHC equipment by the Company
have grown significantly since then.
VasoHealthcare’s
current offerings consist of:
·
GEHC diagnostic imaging capital equipment.
·
GEHC service agreements.
·
GEHC and third party financial services.
VasoHealthcare has built a team of approximately 90 highly
experienced sales professionals who utilize highly focused sales management and
analytic tools to manage the complete sales process and to increase market
penetration.
Vasomedical Global and Vasomedical Solutions
Vasomedical Global was formed in 2011 to combine and
coordinate the various design, development, manufacturing, and sales operations
of medical devices acquired by the Company. These devices primarily consist of
cardiovascular diagnostic and therapeutic systems. Its current offerings consist of:
·
Biox™ series Holter monitors and ambulatory
blood pressure recorders.
·
ARCS™ series analysis, reporting and
communication software for physiological signals such as ECG and blood
pressure.
·
MobiCare™ multi-parameter wireless vital-sign
monitoring system.
·
EECP® therapy system for non-invasive,
outpatient treatment of ischemic heart disease.
This segment uses its extensive cardiovascular device
knowledge coupled with its significant engineering resources to cost-effectively
create and market its proprietary technology. It works with a global
distribution network of channel partners, as well as a global joint venture
arrangement, to sell its products.
NOTE B - BASIS OF PRESENTATION AND
CRITICAL ACCOUNTING POLICIES
Basis of Presentation and Use of
Estimates
The accompanying
condensed consolidated financial statements have been prepared in accordance
with accounting principles generally accepted in the United States of America
("U.S. GAAP") and pursuant to the accounting and disclosure rules and
regulations of the Securities and Exchange Commission (the "SEC").
Certain information and disclosures normally included in the unaudited condensed
consolidated financial statements prepared in accordance with U.S. GAAP have
been condensed or omitted pursuant to such rules and regulations. Accordingly,
these condensed consolidated financial statements should be read in connection
with the audited consolidated financial statements and related notes thereto
included in the Company's Annual Report on Form 10-K for the year ended December
31, 2015, as filed with the SEC on March 30, 2016.
These unaudited condensed
consolidated financial statements include the accounts of the companies over
which we exercise control. In the opinion of management, the accompanying condensed
consolidated financial statements reflect all adjustments (consisting of normal
recurring adjustments) considered necessary for a fair presentation of interim
results for the Company. The results of operations for any interim period are
not necessarily indicative of results to be expected for any other interim
period or the full year.
The
preparation of financial statements in conformity with U.S. GAAP requires
management to make estimates and assumptions that affect the reported amounts
of assets and liabilities as of the date of the condensed
consolidated
financial statements, the disclosure of contingent assets and liabilities in
the unaudited condensed consolidated financial statements and the accompanying
notes, and the reported amounts of revenues, expenses and cash flows during the
periods presented. Actual amounts and results could differ from those
estimates. The estimates and assumptions the Company makes are based on historical
factors, current circumstances and the experience and judgment of the Company's
management. The Company evaluates its estimates and assumptions on an ongoing
basis.
Significant Accounting Policies and Recent Accounting
Pronouncements
During the first quarter of 2016,
we adopted Accounting Standards Update (“ASU”) No. 2015-16, Simplifying the Accounting for
Measurement-period Adjustments, and ASU No. 2016-09, Compensation – Stock Compensation (Topic 718): Improvements to Employee
Share-Based Payment Accounting, neither of which had an impact on our
reported financial position or results of operations and cash flows. There have been no other significant
changes in our reported financial position or results of operations and cash
flows as a result of the adoption of new accounting pronouncements or to our
significant accounting policies that were disclosed in our Annual Report on
Form 10-K for the fiscal year ended December 31, 2015 that have had a
significant impact on our consolidated financial statements or notes thereto.
In April 2016, the FASB issued ASU
No. 2016-10, Revenue from Contracts with
Customers (Topic 606): Identifying Performance Obligations and Licensing,
which adds further guidance on identifying performance obligations and improves
the operability and understanding of the licensing implementation
guidance. The standard is effective
for fiscal periods beginning after December 15, 2017, including interim periods
therein. Early application for
public entities is permitted only as of annual reporting periods beginning
after December 15, 2016, including interim reporting periods within that
reporting period. The Company is
currently evaluating the impact of the adoption of this standard on its
consolidated financial statements.
Variable Interest
Entities
The
Company follows the guidance of accounting for variable interest entities,
which requires certain variable interest entities to be consolidated by the
primary beneficiary of the entities.
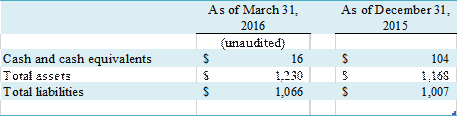
Biox is a Variable Interest Entity (“VIE”).
Liabilities recognized as a result of consolidating this
VIE do not represent additional claims on the Company’s general assets. The
financial information of Biox, which is included in the accompanying condensed consolidated
financial statements, is presented as follows:
(in thousands)
(in thousands)

Reclassifications
Certain reclassifications have
been made to prior period amounts to conform with the current period presentation.
NOTE C – SEGMENT REPORTING AND
CONCENTRATIONS
Vasomedical, Inc. principally operates in three distinct
business segments in the healthcare equipment and information technology
industries. We manage and evaluate
our operations, and report our financial results, through these three business
segments.
·
IT segment, operating through a wholly-owned
subsidiary VasoTechnology, Inc., primarily focuses on healthcare IT and managed
network technology services;
·
Professional sales service segment, operating
through a wholly-owned subsidiary Vaso Diagnostics, Inc. d/b/a VasoHealthcare,
primarily focuses on the sale of healthcare capital equipment for large OEMs
into the health provider middle market; and
·
Equipment segment, operating through
wholly-owned subsidiaries Vasomedical Global Corp. and Vasomedical Solutions,
Inc., primarily focuses on the design, manufacture, sale and service of
proprietary medical devices.
The chief operating decision maker
is the Company’s Chief Executive Officer, who, in conjunction with upper
management, evaluates segment performance based on operating income and
adjusted EBITDA (net income (loss), plus interest expense (income), net; tax
expense; depreciation and amortization; and non-cash stock-based compensation). Administrative functions such as
finance, human resources, and information technology are centralized and
related expenses allocated to each segment. Other costs not directly attributable to
operating segments, such as audit, legal, director fees, investor relations,
and others, as well as certain assets – primarily cash balances – are reported
in the Corporate entity below.
There are no intersegment revenues.
Summary financial information for the segments is set forth below:
(in
thousands) 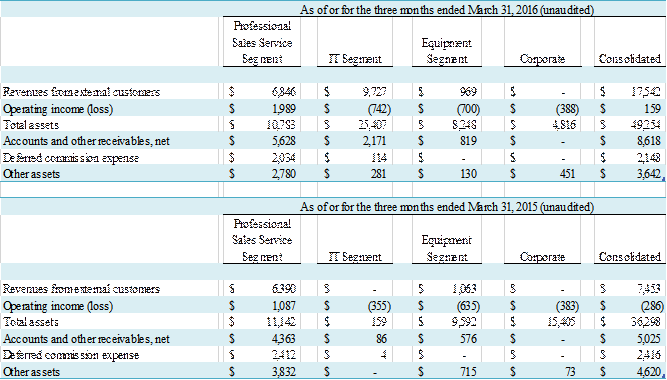
For the three months ended March
31, 2016 and 2015, GE Healthcare accounted for 39% and 86% of revenue,
respectively, and $5.3 million or 62%, and $8.1 million or 69%, of accounts and
other receivables at March 31, 2016 and December 31, 2015, respectively.
NOTE D –LOSS PER COMMON SHARE
Basic loss per common share is computed
as earnings applicable to common stockholders divided by the weighted-average
number of common shares outstanding for the period. Diluted loss per common share reflects
the potential dilution that could occur if securities or other contracts to
issue common shares were exercised or converted to common stock.
The following table represents
common stock equivalents that were excluded from the computation of diluted
earnings per share for the three months ended March 31, 2016 and 2015, because
the effect of their inclusion would be anti-dilutive.
(in thousands)
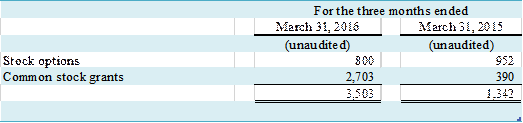
NOTE
E – ACCOUNTS AND OTHER RECEIVABLES, NET
The following table presents information regarding the
Company’s accounts and other receivables as of March 31, 2016 and December 31, 2015:
(in thousands)

Trade receivables include
amounts due for shipped products and services rendered. Amounts currently due under the GEHC Agreement
are subject to adjustment in subsequent periods should the underlying sales
order amount, upon which the receivable is based, change.
Allowance for doubtful
accounts and commission adjustments include estimated losses resulting from the
inability of our customers to make required payments, and adjustments arising
from subsequent changes in sales order amounts that may reduce the amount the
Company will ultimately receive under the GEHC Agreement. Due from employees is primarily
commission advances made to sales personnel.
Inventories,
net of reserves, consist of the following:
(in thousands)
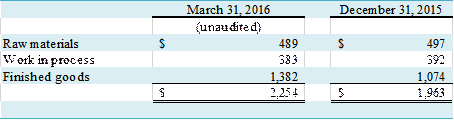
At March 31, 2016 and December
31, 2015, the Company maintained reserves for slow moving inventories of $841,000
and $861,000, respectively.
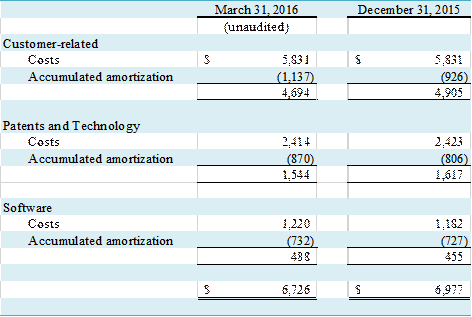
Patents and technology, and
software, are amortized on a straight line basis over their estimated useful
lives of ten, and five years, respectively. The cost of significant customer-related
intangibles is amortized in proportion to estimated total related revenue; cost
of other customer-related intangible assets is amortized on a straight-line basis over the
asset's estimated economic life of seven years. Software costs are
amortized on a straight-line basis over its expected useful life of five
years.
The changes in the Company’s deferred revenues are as
follows:
(in thousands)
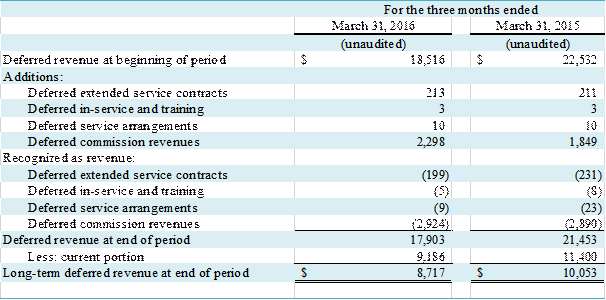
NOTE K – BUSINESS COMBINATION
On May 29, 2015, the Company entered into an agreement for,
and completed its purchase of, all of the assets of NetWolves, LLC and its
affiliates, including the membership interests in NetWolves Network Services
LLC (collectively, “NetWolves”) for
$18,000,000 (the “Purchase Price”). The purchase of NetWolves was accomplished
pursuant to an Asset Purchase Agreement (the "Purchase
Agreement"). As a result, the
Company effectively purchased all rights, titles and ownership of all assets
held by NetWolves. The
Purchase Price was paid using $14,200,000 in cash on hand and $3,800,000 raised
through the issuance of a secured subordinated promissory note (“Note”) to MedTechnology
Investments, LLC (“Medtech” - see Note L).
The Company believes there are significant operational synergies between
NetWolves’ capabilities and VasoHealthcare IT’s requirements under its VAR
contract with GEHC, as well as the opportunity to expand NetWolves’ existing
services to the healthcare IT market.
In accordance with Accounting
Standards Codification 805, Business Combinations, the total purchase
consideration is allocated to the net tangible and intangible assets acquired
and liabilities assumed based on their estimated fair values at May 29, 2015
(the acquisition date). The
following table summarizes the allocation of the assets acquired and
liabilities assumed based on their estimated fair values as follows:
(in thousands)

The goodwill is expected to be deductible for tax purposes.
The
following unaudited supplemental pro forma information presents the financial
results as if the acquisition of NetWolves had occurred January 1, 2014.
(in
thousands)

NOTE L – RELATED-PARTY TRANSACTIONS
One of the Company’s directors, Peter Castle, was the Chief
Executive Officer and President of NetWolves, LLC. Another of the Company’s directors,
David Lieberman, was a director of NetWolves Network Services, LLC. Mr. Castle
and Mr. Lieberman owned of record approximately 10.4% and 5.7%, respectively of
the membership interests of NetWolves LLC.
Mr. Lieberman may also be deemed to have owned beneficially up to an
additional 13.5% of such membership interests. The Company’s board of directors
negotiated the Purchase Price on an arm’s length basis, and both Mr. Castle and
Mr. Lieberman abstained from the vote approving the Purchase Agreement (see
Note K).
The Company obtained an opinion regarding the fairness of the
Purchase Price for NetWolves from a reputable, independent third-party
investment banking firm.
$14,200,000 of the Purchase Price was paid for by cash on hand, and the
remaining $3,800,000 was raised from the sale of the Note to MedTech. Of the $4,800,000 borrowed from MedTech,
$2,200,000 was provided by six of our directors or members of their families,
and an additional $100,000 was provided by an additional director prior to his
joining the board of directors in June 2015. The MedTech Note bears interest at
9% per annum.
David Lieberman, the Vice Chairman of the Company’s Board of
Directors, is a practicing attorney in the State of New York and a senior
partner at the law firm of Beckman, Lieberman & Barandes, LLP, which
performs certain legal services for the Company. Fees of approximately $85,000 and $60,000
were billed by the firm for the three-month periods ended March 31, 2016 and
2015, respectively, at which date no amounts were outstanding.
In January 2015, operations began
under the VSK joint venture. The
Company accounts for its investment in VSK using the equity method. At March 31, 2016, the Company had
contributed $200,000 to VSK, and $87,000 was due from VSK for equipment and
services the Company billed to it. The Company’s noncontrolling interest in
VSK’s loss from operations approximated $73,000 and $2,000 for the three-month
periods ended March 31, 2016 and 2015, respectively.
NOTE M – COMMITMENTS AND CONTINGENCIES
Litigation
The Company is currently, and has been in the past, a
party to various routine legal proceedings, primarily employee related matters,
incident to the ordinary course of business. The Company believes that the
outcome of all such pending legal proceedings in the aggregate is unlikely to
have a material adverse effect on the business or consolidated financial
condition of the Company.
Sales
representation agreement
In June
2012, the Company concluded an amendment of the GEHC Agreement with GEHC,
originally signed on May 19, 2010.
The amendment, effective July 1, 2012, extended the initial term of
three years commencing July 1, 2010 to five years through June 30, 2015. In December 2014, the Company concluded
an additional amendment, effective January 1, 2015, extending the term through
December 31, 2018, subject to earlier termination under certain circumstances
and termination without cause on or after July 1, 2017. These circumstances include not
materially achieving certain sales goals, not maintaining a minimum number of
sales representatives, and various legal and GEHC policy requirements. Under the terms of the agreement, the
Company is required to lease dedicated computer equipment from GEHC for
connectivity to their network.
NOTE N – SUBSEQUENT EVENT
In April
2015, the Company issued approximately 1.1 million shares of restricted common
stock to an employee in satisfaction of a compensation liability.
Except for
historical information contained in this report, the matters discussed are
forward-looking statements that involve risks and uncertainties. When used in
this report, words such as “anticipates”, “believes”, “could”, “estimates”,
“expects”, “may”, “plans”, “potential” and “intends” and similar expressions,
as they relate to the Company or its management, identify forward-looking
statements. Such forward-looking statements are based on the beliefs of the
Company’s management, as well as assumptions made by and information currently
available to the Company’s management. Among the factors that could cause
actual results to differ materially are the following: the effect of business and economic conditions; the effect of the dramatic changes taking place in the
healthcare environment; the impact of competitive procedures and products and
their pricing; medical insurance reimbursement policies; unexpected
manufacturing or supplier problems; unforeseen difficulties and delays in the
conduct of clinical trials and other product development programs; the actions
of regulatory authorities and third-party payers in the United States and
overseas; continuation of the GEHC agreements and the risk factors reported
from time to time in the Company’s SEC reports, including its recent report on
Form 10-K. The Company undertakes
no obligation to update forward-looking statements as a result of future events
or developments.
Unless the context
requires otherwise, all references to “we”, “our”, “us”, “Company”,
“registrant”, “Vasomedical” or “management” refer to Vasomedical, Inc. and its
subsidiaries
General Overview
Vasomedical, Inc. (“Vasomedical”)
was incorporated in Delaware in July 1987. We principally operate in three
distinct business segments in the healthcare equipment and information
technology industries. We manage
and evaluate our operations, and report our financial results, through these
three business segments.
·
IT segment, operating through a wholly-owned
subsidiary VasoTechnology, Inc., primarily focuses on healthcare IT and managed
network technology services;
·
Professional sales service segment, operating
through a wholly-owned subsidiary Vaso Diagnostics, Inc. d/b/a VasoHealthcare,
primarily focuses on the sale of healthcare capital equipment for large OEMs
into the health provider middle market; and
·
Equipment segment, operating through
wholly-owned subsidiaries Vasomedical Global Corp. and Vasomedical Solutions,
Inc., primarily focuses on the design, manufacture, sale and service of
proprietary medical devices.
VasoTechnology
VasoTechnology, Inc. was
formed in May 2015, at the time the Company acquired all
of the assets of NetWolves, LLC and its affiliates, including the membership
interests in NetWolves Network Services LLC (collectively, “NetWolves”), to
address a major issue facing the healthcare IT industry. It currently consists of a managed
network and security service division and a healthcare IT application VAR
(value added reseller) division. Its
current offering includes:
·
Managed diagnostic imaging applications
(national channel partner of GEHC IT).
·
Managed network infrastructure (routers,
switches and other core equipment).
·
Managed network transport (FCC licensed carrier
reselling 175+ facility partners).
·
Managed security services (IBM’s first security
white label partner).
VasoTechnology uses a combination of proprietary technology,
methodology and best-in-class third-party applications to deliver its value
proposition.
VasoHealthcare
VasoHealthcare commenced operations in 2010, in conjunction
with the Company’s execution of its exclusive sales representation agreement
with General Electric Healthcare (“GEHC”), which is the healthcare business
division of the General Electric Company (“GE”), to exploit the sale of certain
healthcare capital equipment in the health provider middle market. Sales of GEHC equipment by the Company
have grown significantly since then.
VasoHealthcare’s
current offering consists of:
·
GEHC diagnostic imaging capital equipment.
·
GEHC service agreements.
·
GEHC and third party financial services.
VasoHealthcare has built a team of approximately 90 highly
experienced sales professionals who utilize highly focused sales management and
analytic tools to manage the complete sales process and to increase market
penetration.
Vasomedical Global and Vasomedical Solutions
Vasomedical Global was formed in 2011 to combine and
coordinate the various design, development, manufacturing, and sales operations
of medical devices acquired by the Company. These devices primarily consist of
cardiovascular diagnostic and therapeutic systems. Its current offering consists of:
·
Biox™ series Holter monitors and ambulatory
blood pressure recorders .
·
ARCS™ series analysis, reporting and communication
software for physiological signals such as ECG and blood pressure.
·
MobiCare™ multi-parameter wireless vital-sign
monitoring system.
·
EECP® therapy systems, used for
non-invasive, outpatient treatment of ischemic heart disease.
This segment uses its extensive cardiovascular device
knowledge coupled with its significant engineering resources to cost-effectively
create and market its proprietary technology. It works with a global
distribution network of channel partners, as well as a global joint venture
arrangement, to sell its products.
Critical Accounting Policies and Estimates
Our discussion and analysis of
our financial condition and results of operations are based upon the
accompanying unaudited condensed consolidated financial statements, which have
been prepared in accordance with accounting principles generally accepted in
the United States (“U.S. GAAP”). The preparation of financial statements in
conformity with U.S. GAAP requires management to make judgments, estimates and
assumptions that affect the reported amounts of assets, liabilities, revenue,
expenses, and the related disclosures at the date of the financial statements
and during the reporting period. Although these estimates are based on our
knowledge of current events, our actual amounts and results could differ from
those estimates. The estimates made are based on historical factors, current
circumstances, and the experience and judgment of our management, who
continually evaluate the judgments, estimates and assumptions and may employ
outside experts to assist in the evaluations.
Certain of our accounting
policies are deemed “critical”, as they are both most important to the
financial statement presentation and require management’s most difficult,
subjective or complex judgments as a result of the need to make estimates about
the effect of matters that are inherently uncertain. For a discussion of our
critical accounting policies, see “Management’s Discussion and Analysis of
Financial Condition and Results of Operations” in our Annual Report on Form 10-K
for the year ended December 31, 2015 as filed with the SEC on March 30, 2016.
Results of Operations – For the Three Months Ended March 31,
2016 and 2015
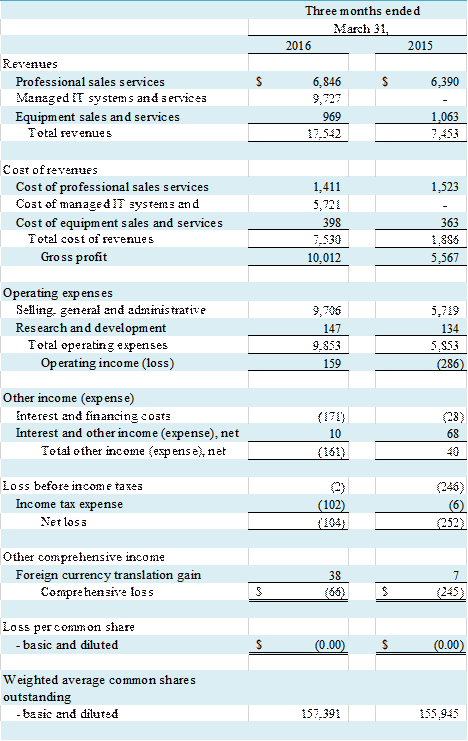
Total revenue for the three months
ended March 31, 2016 and 2015 was $17,542,000 and $7,453,000, respectively, representing
an increase of $10,089,000, or 135% year-over-year. The revenue increase was primarily due to
$9,727,000 in revenue in the IT segment, of which $9,238,000 resulted from the operations
of NetWolves, which the Company acquired at the end of May 2015. Net loss for the three months ended March
31, 2016 was $104,000, compared to a net loss of $252,000 for the three months
ended March 31, 2015, representing an improvement of $148,000. Our total net loss was $0.00 per basic
and diluted common share for the three months ended March 31, 2016 and 2015.
Revenues
Commission revenues in the professional
sales services segment were $6,846,000 in the first quarter of 2016, an
increase of 7%, as compared to $6,390,000 in the first quarter of 2015. The increase in commission revenues in
the first quarter of 2016 was due primarily to an increase in equipment
delivered by GEHC during the quarter, partially offset by lower commission
rates on such deliveries. The
Company recognizes commission revenue when the underlying equipment has been
accepted at the customer site in accordance with the specific terms of the
sales agreement. Consequently,
amounts billable under the agreement with GE Healthcare prior to customer
acceptance of the equipment are recorded as deferred revenue in the condensed
consolidated balance sheet. As of March
31, 2016, $16,744,000 in deferred commission revenue was recorded in the
Company’s condensed consolidated balance sheet, of which $8,206,000 was long-term. At March 31, 2015, $20,114,000 in
deferred commission revenue was recorded in the Company’s condensed
consolidated balance sheet, of which $9,420,000 was long-term.
Revenue in the IT segment for
the three months ended March 31, 2016 was $9,727,000 compared to $0 revenue for
the three months ended March 31, 2015, of which $9,238,000 resulted from the
acquisition of NetWolves in the second quarter 2015, and $489,000 resulted from
growth in the healthcare IT VAR business.
Revenue in our equipment segment
decreased by $94,000, or 9%, to $969,000 for the three-month period ended March
31, 2016 compared to the same period of the prior year. The decrease was principally due to a
decrease in EECP® revenues and international sales by our China
operations as a result of lower sales volume.
Gross Profit
The Company had a gross profit
of $10,012,000, or 57% of revenue, in the first quarter of 2016 compared to $5,567,000,
or 75% of revenue, in the first quarter of the prior year, an increase of $4,445,000,
or 80%. The increase is principally
due to $4,006,000 in gross profit in the IT segment, of which $3,883,000
resulted from the acquisition of NetWolves, combined with higher revenues and gross
profit margin in the professional sales services segment.
Professional sales services
segment gross profit was $5,435,000, or 79% of the segment revenue, for the three
months ended March 31, 2016 as compared to $4,867,000, or 76% of the segment
revenue, for the three months ended March 31, 2015. The increase in absolute dollars and
margin percentage was due to higher delivery volume of GEHC equipment during
the first quarter of 2016 than in the same period last year, as well as lower commission expense in the first quarter
of 2016. Cost of commissions of $1,411,000
and $1,523,000, for the three months ended March 31, 2016 and 2015,
respectively, reflected commission expense associated with recognized
commission revenues. The decrease
was due to lower commission expense rates on certain deliveries in the first quarter
2016 compared to the same period in 2015. Commission expense associated with
deferred revenue is recorded as deferred commission expense until the related
commission revenue is recognized.
IT segment gross profit for the
three months ended March 31, 2016 was $4,006,000, or 41% of revenue, compared
to $0 gross profit for the three months ended March 31, 2015, with the increase
primarily resulting from the acquisition of NetWolves.
Equipment segment gross profit decreased
to $571,000, or 59% of equipment segment revenues, for the first quarter of 2016
compared to $700,000, or 66% of equipment segment revenues, for the same
quarter of 2015. Gross profit margin
in the equipment segment decreased due mainly to lower average selling prices.
Operating Income (Loss)
Operating income was $159,000
for the three months ended March 31, 2016, compared to an operating loss of $286,000
for the three months ended March 31, 2015, an improvement of $445,000. The
improvement in operating income is due to the increase in gross profit and
lower selling, general and administrative, or SG&A, costs relative to
revenue. SG&A costs were 55% of
revenue for the three months ended March 31, 2016, compared to 77% of revenue
for the three months ended March 31, 2015. Operating income in the professional
sales services segment increased by $902,000 to $1,989,000, compared to $1,087,000
in the first quarter of the prior year, due mainly to a higher gross margin
combined with lower SG&A costs. In addition, operating loss in the equipment
segment increased by $65,000, or 10%, to $700,000 compared to $635,000 in the
same quarter of the prior year, due primarily to a one-time charge of $412,000,
included in SG&A expense, to reserve a loan receivable, and $129,000 lower
gross profit, partially offset by a $487,000 decrease in all other SG&A
costs in the current year quarter. Our
IT segment had an operating loss of $742,000 in the first quarter of 2016 as
compared to an operating loss of $355,000 in the same quarter of the prior year. The increase of $387,000 was primarily due
to an increase of $349,000 attributable to the inclusion of NetWolves.
SG&A expenses for the first quarter
of 2016 and 2015 were $9,706,000, or 55% of revenues, and $5,719,000, or 77% of
revenues, respectively, reflecting an increase of $3,987,000 or approximately 70%.
The increase in SG&A expenditures in the first quarter of 2016 resulted
primarily from $4,749,000 in costs attributable to the IT segment in 2016 mainly
due to the inclusion of NetWolves operations during the quarter, and higher sales
and marketing expenses in the GEHC VAR business, partially offset by lower
costs in the professional sales services and equipment segments. Despite the inclusion of a one-time
charge of $412,000 recorded in the first quarter of 2016 related to the
reserving of a loan receivable, equipment segment SG&A decreased to
$1,125,000 in the first quarter of 2016 from $1,200,000 in the same period of
2015.
Research
and development (“R&D”) expenses were $147,000, or 1% of revenues (15% of
Equipment segment revenues), for the first quarter of 2016, an increase of $13,000,
or 10%, from $134,000, or 2% of revenues (13% of Equipment segment revenues),
for the first quarter of 2015. The increase is primarily attributable to higher
product development expenses in the first quarter of 2016.
Adjusted EBITDA
We define Adjusted
EBITDA (earnings (loss) before interest, taxes, depreciation and amortization),
which is a non-GAAP financial measure, as net income (loss), plus interest expense
(income), net; tax expense; depreciation and amortization; and non-cash
expenses for share-based compensation. Adjusted EBITDA is a metric that
is used by the investment community for comparative and valuation
purposes. We disclose this metric
in order to support and facilitate the dialogue with research analysts and
investors.
Adjusted EBITDA is
not a measure of financial performance under accounting principles generally
accepted in the United States (“GAAP”) and should not be considered a substitute
for operating income, which we consider to be the most directly comparable GAAP
measure. Adjusted EBITDA has limitations as an analytical tool, and when
assessing our operating performance, you should not consider Adjusted EBITDA in
isolation, or as a substitute for net income or other consolidated income
statement data prepared in accordance with GAAP. Other companies may calculate
Adjusted EBITDA differently than we do, limiting its usefulness as a
comparative measure.
A reconciliation of net loss to Adjusted EBITDA is set forth
below:
(in thousands)
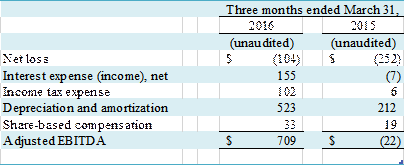
Adjusted EBITDA increased by $731,000 to $709,000 in the quarter
ended March 31, 2016 from $(22,000) in the quarter ended March 31, 2015. The increase was primarily attributable
to the lower net loss and higher fixed asset depreciation in the IT segment and
amortization of intangibles associated with the NetWolves acquisition in May
2015, as well as higher interest expense arising from the MedTech Note used to
partially fund the NetWolves acquisition and higher income tax expense.
Interest and Other Income (Expense)
Interest and other income
(expense) for the first quarter of 2016 was $(161,000) as compared to $40,000
for the first quarter of 2015. The change from income to expense was due primarily
to higher interest expense from debt associated with the Genwell and NetWolves
acquisitions.
Income Tax Expense
During
the first quarter of 2016 we recorded income tax expense of $102,000 as compared
to income tax expense of $6,000 for the first quarter of 2015. The increase arose from higher U.S.
income taxes associated with tax amortization arising from the NetWolves
purchase.
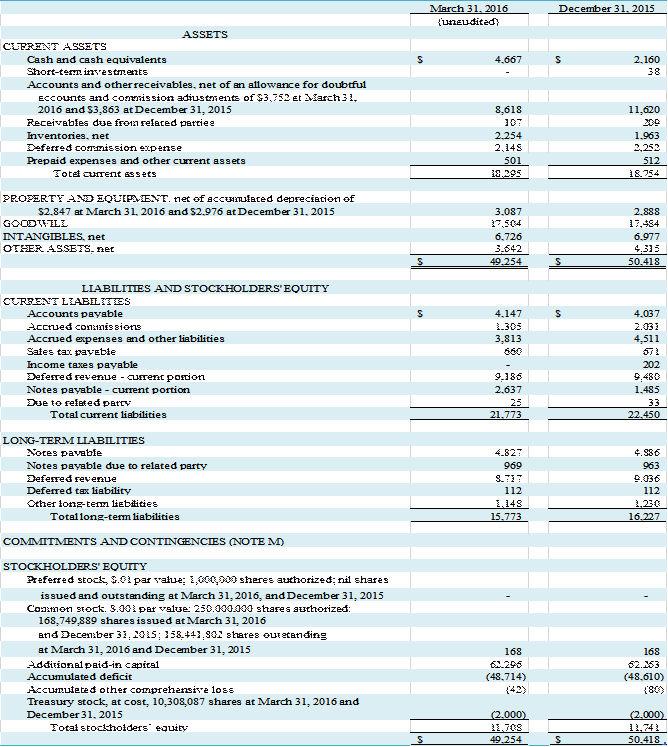
Liquidity and Capital Resources
Cash and Cash Flow
We have financed our operations
from working capital. At March 31, 2016,
we had cash and cash equivalents of $4,667,000 and negative working capital of
$3,478,000 compared to cash and cash equivalents of $2,160,000, short-term
investments of $38,000 and negative working capital of $3,696,000 at December
31, 2015. $7,038,000 in negative
working capital at March 31, 2016 is attributable to the net balance of deferred
commission expense and deferred revenue.
These are non-cash expense and revenue items and have no impact on
future cash flows.
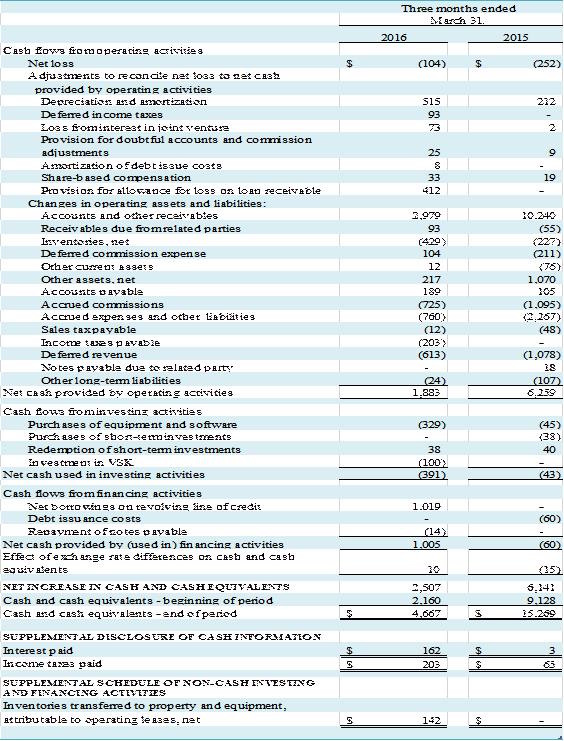
Cash provided by operating
activities was $1,883,000 during the first quarter of 2016, compared to $6,259,000
for the same period in 2015, which consisted of net loss after adjustments to
reconcile net loss to net cash of $1,055,000 and cash provided by operating
assets and liabilities of $828,000. The changes in the account balances
primarily reflect a decrease in accounts and other receivables of $2,979,000, partially
offset by decreases in deferred revenue of $613,000, accrued expenses of $760,000,
and accrued commissions of $725,000.
Significantly higher commission billings and recognized revenue were generated
in the fourth quarter of 2014, resulting in significant cash inflows early in 2015.
Cash used in investing activities during the three-month
period ended March 31, 2016 was $391,000.
We invested $100,000 in the VSK joint venture as part of our capital
contribution, and, $329,000 was used for the purchase of equipment and software. This was partially offset by the
redemption of short-term investments of $38,000.
Cash provided by financing activities during the three-month
period ended March 31, 2016 was $1,005,000 as a result of borrowings on our
line of credit, partially offset by $14,000 in repayments of notes issued for
equipment purchases.
Liquidity
The Company
expects to be profitable for the year ending December 31, 2016 and to continue to
generate positive cash flow through its existing professional sales services
operations, from the operation of NetWolves, and improved operating efficiency
and growth in its China operations and by expanding its market presence and
product portfolio. The Company has
reorganized its EECP® business model, both domestically and
internationally, including the start of operations of the joint venture VSK
Medical, intended to reduce costs and achieve profitability in this business. The Company will continue to pursue
acquisitions and partnership opportunities in the international and domestic
markets and will look to expand its sales representation business.
Evaluation of
Disclosure Controls and Procedures
Disclosure controls and procedures reporting as
promulgated under the Exchange Act is defined as controls and procedures that
are designed to ensure that information required to be disclosed by us in the
reports that we file or submit under the Exchange Act are recorded, processed,
summarized and reported within the time periods specified in the SEC rules and
forms. Disclosure controls and
procedures include without limitation, controls and procedures designed to
ensure that information required to be disclosed by us in the reports that we
file or submit under the Exchange Act is accumulated and communicated to our
management, including our Chief Executive Officer (“CEO”) and Chief Financial
Officer (“CFO”), or persons performing similar functions, as appropriate to
allow timely decisions regarding required disclosure.
Our CEO and our CFO have evaluated the effectiveness of the
design and operation of our disclosure controls and procedures as of March 31, 2016
and have concluded that the Company’s disclosure controls and procedures were
effective as of March 31, 2016.
Changes in
Internal Control Over Financial Reporting
There
was no change in the Company’s internal control over financial reporting during
the Company’s last fiscal quarter that has materially affected, or is
reasonably likely to materially affect, the Company’s internal control over
financial reporting.
Exhibits
31 Certifications of the
Chief Executive Officer and the Chief Financial Officer pursuant to Rules
13a-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
32 Certifications
of the Chief Executive Officer and the Chief Financial Officer pursuant to 18
U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley
Act of 2002.
In accordance with the
requirements of the Exchange Act, the Registrant caused this report to be
signed on its behalf by the undersigned, thereunto duly authorized.
VASOMEDICAL,
INC.
By: /s/ Jun Ma
Jun Ma
President
and Chief Executive Officer
(Principal
Executive Officer)
/s/ Michael J. Beecher .
Michael
J. Beecher
Chief
Financial Officer and Principal Accounting Officer
Date: May 13, 2016
EXHIBIT 31.1
CERTIFICATION
PURSUANT TO RULE 13a/15d OF THE SECURITIES EXCHANGE ACT OF 1934,
AS
ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Jun Ma, certify that:
- I have reviewed this quarterly report on Form 10-Q of
Vasomedical, Inc. and subsidiaries (the “registrant”);
- Based on my knowledge, this report does not contain
any untrue statement of a material fact or omit to state a material fact
necessary to make the statements made, in light of the circumstances under
which such statements were made, not misleading with respect to the period
covered by this report;
- Based on my knowledge, the financial statements, and
other financial information included in this report, fairly present in all
material respects the financial condition, results of operations and cash
flows of the registrant as of, and for, the periods presented in this
report;
- The registrant’s other certifying officer and I are
responsible for establishing and maintaining disclosure controls and
procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial
reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for
the registrant and have:
- Designed such disclosure controls and procedures, or
caused such disclosure controls and procedures to be designed under our
supervision, to ensure that material information relating to the
registrant, including its consolidated subsidiaries, is made known to us
by others within those entities, particularly during the period in which
this report is being prepared;
- Designed such internal control over financial
reporting, or caused such internal control over financial reporting to be
designed under our supervision, to provide reasonable assurance regarding
the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted
accounting principles;
- Evaluated the effectiveness of the registrant’s
disclosure controls and procedures and presented in this report our
conclusions about the effectiveness of the disclosure controls and
procedures, as of the end of the period covered by this report based on
such evaluation; and
- Disclosed in this report any change in the
registrant’s internal control over financial reporting that occurred
during the registrant’s most recent fiscal quarter (the registrant’s
fourth fiscal quarter in the case of an annual report) that has
materially affected, or is reasonably likely to materially affect, the
registrant’s internal control over financial reporting; and
- The registrant’s other certifying officer and I have
disclosed, based on our most recent evaluation of internal control over
financial reporting, to the registrant’s auditors and the audit committee
of the registrant’s Board of Directors (or persons performing the
equivalent functions):
- All significant deficiencies and material weaknesses
in the design or operation of internal control over financial reporting
which are reasonably likely to adversely affect the registrant’s ability
to record, process, summarize, and report financial information; and
- Any fraud, whether or not material, that involves
management or other employees who have a significant role in the
registrant’s internal control over financial reporting.
/s/ Jun Ma
.
Jun Ma
President and Chief Executive Officer
Date: May 13, 2016
EXHIBIT 31.2
CERTIFICATION
PURSUANT TO RULE 13a/15d OF THE SECURITIES EXCHANGE ACT OF 1934,
AS
ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Michael J. Beecher, certify
that:
- I have reviewed this quarterly report on Form 10-Q of
Vasomedical, Inc. and subsidiaries (the “registrant”);
- Based on my knowledge, this report does not contain
any untrue statement of a material fact or omit to state a material fact
necessary to make the statements made, in light of the circumstances under
which such statements were made, not misleading with respect to the period
covered by this report;
- Based on my knowledge, the financial statements, and
other financial information included in this report, fairly present in all
material respects the financial condition, results of operations and cash
flows of the registrant as of, and for, the periods presented in this
report;
- The registrant’s other certifying officer and I are
responsible for establishing and maintaining disclosure controls and
procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial
reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for
the registrant and have:
- Designed such disclosure controls and procedures, or
caused such disclosure controls and procedures to be designed under our
supervision, to ensure that material information relating to the
registrant, including its consolidated subsidiaries, is made known to us
by others within those entities, particularly during the period in which
this report is being prepared;
- Designed such internal control over financial
reporting, or caused such internal control over financial reporting to be
designed under our supervision, to provide reasonable assurance regarding
the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted
accounting principles;
- Evaluated the effectiveness of the registrant’s
disclosure controls and procedures and presented in this report our
conclusions about the effectiveness of the disclosure controls and
procedures, as of the end of the period covered by this report based on
such evaluation; and
- Disclosed in this report any change in the registrant’s
internal control over financial reporting that occurred during the
registrant’s most recent fiscal quarter (the registrant’s fourth fiscal
quarter in the case of an annual report) that has materially affected, or
is reasonably likely to materially affect, the registrant’s internal
control over financial reporting; and
- The registrant’s other certifying officer and I have
disclosed, based on our most recent evaluation of internal control over
financial reporting, to the registrant’s auditors and the audit committee
of the registrant’s Board of Directors (or persons performing the
equivalent functions):
- All significant deficiencies and material weaknesses
in the design or operation of internal control over financial reporting
which are reasonably likely to adversely affect the registrant’s ability
to record, process, summarize, and report financial information; and
- Any fraud, whether or not material, that involves
management or other employees who have a significant role in the
registrant’s internal control over financial reporting.
/s/ Michael J. Beecher
.
Michael J. Beecher
Chief Financial Officer
Date: May 13, 2016
EXHIBIT
32.1
CERTIFICATION
PURSUANT TO
18
U.S.C. SECTION 1350,
AS
ADOPTED PURSUANT TO
SECTION
906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the quarterly
report of Vasomedical, Inc. and subsidiaries (the “Company”) on Form 10-Q for
the period ending March 31, 2016, as filed with the Securities and Exchange
Commission on the date hereof (the “Report”), I, Jun Ma, as President and Chief
Executive Officer of the Company, certify, pursuant to 18 U.S.C. § 1350, as
adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1) The Report fully
complies with the requirements of section 13(a) or 15(d) of the Securities
Exchange Act of 1934, as amended; and
(2) The information
contained in the Report fairly presents, in all material respects, the
financial condition and results of operations of the Company.
/s/ Jun Ma
.
Jun
Ma
President
and Chief Executive Officer
Dated: May 13, 2016
EXHIBIT
32.2
CERTIFICATION
PURSUANT TO
18
U.S.C. SECTION 1350,
AS
ADOPTED PURSUANT TO
SECTION
906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the
quarterly report of Vasomedical, Inc. and subsidiaries (the “Company”) on Form
10-Q for the period ending March 31, 2016, as filed with the Securities and
Exchange Commission on the date hereof (the “Report”), I, Michael J. Beecher,
as Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. §
1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1) The Report fully
complies with the requirements of section 13(a) or 15(d) of the Securities
Exchange Act of 1934, as amended; and
(2) The information
contained in the Report fairly presents, in all material respects, the
financial condition and results of operations of the Company.
/s/ Michael J. Beecher
.
Michael J. Beecher
Chief Financial Officer
Dated: May 13, 2016
![]()