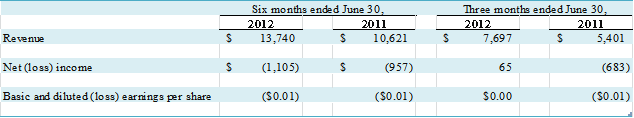NOTE A - ORGANIZATION AND PLAN
OF OPERATIONS
Vasomedical, Inc. was
incorporated in Delaware in July 1987. Unless the context requires otherwise,
all references to “we”, “our”, “us”, “Company”, “registrant”, “Vasomedical” or
“management” refer to Vasomedical, Inc. and its subsidiaries. Until 2010, we were
primarily engaged in designing, manufacturing, marketing and supporting EECP®
enhanced external counterpulsation systems based on
our unique proprietary technology currently indicated for use in cases of
stable or unstable angina (i.e., chest pain), congestive heart failure (“CHF”),
acute myocardial infarction (i.e., heart attack, (MI)) and cardiogenic
shock. In May 2010, the Company, through
its wholly-owned subsidiary Vaso Diagnostics, Inc. d/b/a VasoHealthcare, expanded
into the sales representation business via its agreement with GE Healthcare
(“GEHC”), the healthcare business unit of General Electric Company (NYSE: GE), to
be GEHC’s exclusive sales representative for the sale of select GEHC diagnostic
imaging products in specific market segments in the 48 contiguous states of the
United States and the District of Columbia.
In June 2012, the Company entered
into an amendment, effective July 1, 2012, of the sales representative
agreement (“GEHC Agreement”) extending the initial term of three years
commencing July 1, 2010 to five years through June 30, 2015, subject to earlier
termination under certain circumstances.
In September 2011, the Company acquired Fast Growth
Enterprises Limited (FGE), a British Virgin Islands company which, through its
subsidiaries, owns and controls two Chinese operating companies - Life
Enhancement Technologies Ltd. and Biox Instruments Co.
Ltd., respectively – to expand its technical and manufacturing capabilities and
to enhance its distribution network, technology, and product portfolio. Also in September 2011, the Company
restructured to further align its business management structure and long-term
growth strategy and now operates through three wholly-owned subsidiaries. Vaso Diagnostics
d/b/a VasoHealthcare continues as the operating subsidiary for the sales
representation of GE Healthcare diagnostic imaging products; Vasomedical Global
Corp. operates the Company’s recently-acquired Chinese companies; and
Vasomedical Solutions, Inc. manages and coordinates our EECP® therapy
business as well as other medical equipment operations.
We report the operations of Vasomedical Global Corp. and
Vasomedical Solutions, Inc. under our Equipment reportable segment. VasoHealthcare activities are included under
our Sales Representation reportable segment (See Note C).
NOTE B - BASIS OF PRESENTATION AND
CRITICAL ACCOUNTING POLICIES
Basis of Presentation and Use of
Estimates
The accompanying
consolidated condensed financial statements have been prepared in accordance
with accounting principles generally accepted in the United States of America
("U.S. GAAP") and pursuant to the accounting and disclosure rules and
regulations of the Securities and Exchange Commission (the "SEC").
Certain information and disclosures normally included in the consolidated
condensed financial statements prepared in accordance with U.S. GAAP have been
condensed or omitted pursuant to such rules and regulations. Accordingly, these
consolidated condensed financial statements should be read in connection with
the audited consolidated financial statements and related notes thereto
included in the Company's Transition Report on Form 10-K for the transition
period ended December 31, 2011, as filed with the SEC. These consolidated
condensed financial statements include the accounts of the companies over which
we exercise control. In the opinion of management, the accompanying
consolidated condensed financial statements reflect all adjustments (consisting
of normal recurring adjustments) considered necessary for a fair presentation
of interim results for the Company. The results of operations for any interim
period are not necessarily indicative of results to be expected for any other
interim period or the full year.
The
preparation of financial statements in conformity with U.S. GAAP requires
management to make estimates and assumptions that affect the reported amounts
of assets and liabilities as of the date of the consolidated condensed
financial statements, the disclosure of contingent assets and liabilities in
the consolidated condensed financial statements and the accompanying notes, and
the reported amounts of revenues, expenses and cash flows during the periods
presented. Actual amounts and results could differ from those estimates. The
estimates and assumptions the Company makes are based on historical factors,
current circumstances and the experience and judgment of the Company's
management. The Company evaluates its estimates and assumptions on an ongoing
basis.
Significant Accounting Policies
Note B of the Notes to
Consolidated Financial Statements, included in the Transition Report on Form
10-K for the seven months ended December 31, 2011, includes a summary of the
significant accounting policies used in the preparation of the consolidated condensed
financial statements.
Reclassifications
Certain reclassifications have
been made to prior period amounts to conform with the
current period presentation.
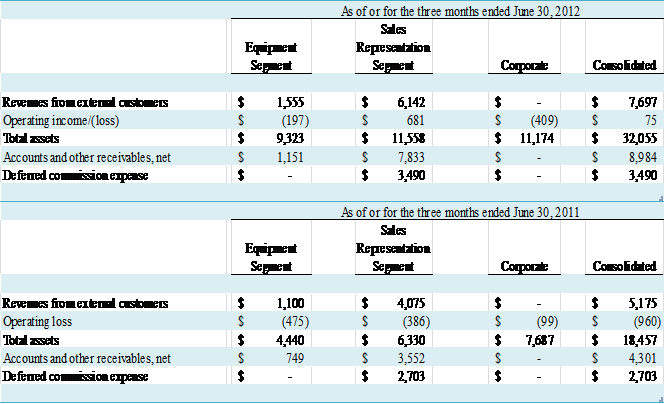
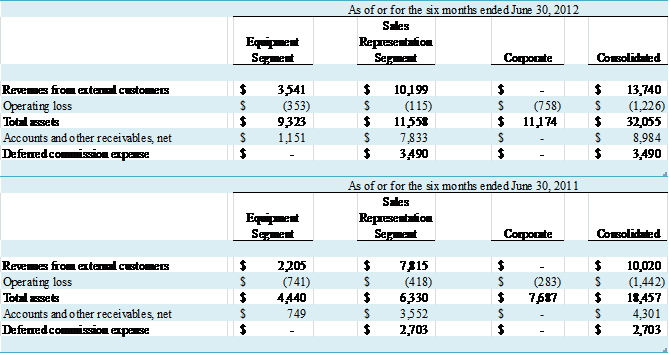
NOTE C – SEGMENT REPORTING AND
CONCENTRATIONS
The Company views its business in two segments – the Equipment
segment and the Sales Representation segment.
The Equipment segment is engaged in designing, manufacturing, marketing
and supporting EECP® enhanced external counterpulsation
systems both domestically and internationally, as well as the marketing of
other medical devices. The Sales Representation
segment operates through the VasoHealthcare subsidiary and is currently engaged
solely in the execution of the Company’s responsibilities under our agreement
with GEHC. The Company evaluates segment
performance based on operating income.
Administrative functions such as finance, human resources, and
information technology are centralized and related expenses allocated to each
segment. Other costs not directly
attributable to operating segments, such as audit, legal, director fees,
investor relations, and others, as well as certain assets – primarily cash
balances – are reported in the Corporate entity below. There are no intersegment revenues. Summary financial information for the
segments is set forth below:
(in thousands)
(in thousands)
For the three months ended June 30,
2012 and 2011, GE Healthcare accounted for 80% and 79% of revenue,
respectively. For the six months ended
June 30, 2012 and 2011, GE Healthcare accounted for 74% and 78% of revenue,
respectively. Also, GE Healthcare accounted for $7.7 million,
or 86%, and $19.7 million, or 95%, of accounts and other receivables at June 30,
2012 and December 31, 2011, respectively.
NOTE D – SHARE-BASED COMPENSATION
The Company complies with ASC
Topic 718 “Compensation – Stock Compensation” (“ASC 718”), which requires all
share-based awards to employees, including grants of employee stock options, to
be recognized in the consolidated condensed financial statements based on their
estimated fair values.
During the six-month period
ended June 30, 2012, the
Company granted 500,000 restricted shares of common stock, valued at $120,000
to an officer, of which half vested immediately and the remainder one year
thereafter. During the three months
ended June 30, 2011, 166,279 shares of restricted common stock valued at
$73,500 were granted to directors, and 366,279 shares of restricted common
stock valued at $135,500 were granted to directors during the six months ended
June 30, 2011.
During the six-month periods
ended June 30, 2012 and 2011,
the Company did not grant any stock options.
For the three and six months
ended June 30, 2012, the Company granted 2,190,000 shares of restricted common
stock valued at $548,000 to non-officer employees in its VasoHealthcare
subsidiary in conjunction with the extension of the GEHC Agreement in June
2012. For the three and six months ended
June 30, 2011, the Company granted 10,000 shares of restricted common stock
valued at $6,800 to non-officer employees.
Share-based compensation expense
recognized for the three and six months ended June 30, 2012 was $102,000 and
$231,000, respectively, and $77,000 and $203,000 for the three
and six months ended June 30, 2011,
respectively. These expenses are
included in cost of revenues; selling, general, and administrative expenses;
and research and development expenses in the consolidated condensed statements
of operations. Expense for share-based
arrangements was $136,000 and $288,000 for the three and six months ended June
30, 2012, respectively, and $162,000 and $208,000 for the three and six months
ended June 30, 2011, respectively.
Unrecognized expense related to existing share-based arrangements is
approximately $1.3 million at June 30, 2012 and will be recognized through July
2013.
NOTE E – EARNINGS (LOSS) PER
COMMON SHARE
Basic
earnings per common share is computed as earnings applicable to common
stockholders divided by the weighted-average number of common shares
outstanding for the period. Diluted earnings per common share reflects the potential
dilution that could occur if securities or other contracts to issue common
shares were exercised or converted to common stock.
Basic and diluted (loss)
per common share was ($0.01) for the six months ended June 30, 2012 and 2011. Basic and diluted earnings (loss) per share
for the three months ended June 30, 2012 and 2011 were $0.00 and ($0.01),
respectively.
Diluted earnings per share were
computed based on the weighted average number of shares outstanding plus all
potentially dilutive common stock equivalents.
A reconciliation of basic to diluted shares used in the earnings per
share calculation is as follows:
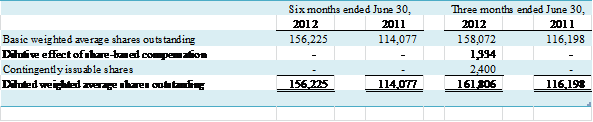
The following table represents
common stock equivalents that were excluded from the computation of diluted
earnings per share for the six and three months ended June 30, 2012 and 2011,
because the effect of their inclusion would be anti-dilutive.
(in thousands)
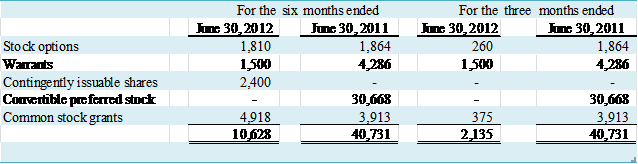
NOTE
F – FAIR VALUE MEASUREMENTS
The Company complies with the provisions of ASC 820 “Fair
Value Measurements and Disclosures” (“ASC 820”). Under ASC 820, fair value is defined as the
price that would be received to sell an asset or paid to transfer a liability
(i.e., the “exit price”) in an orderly transaction between market participants
at the measurement date.
The following tables present information
about the Company’s assets and liabilities measured at fair value as of June 30, 2012 and December 31, 2011
(in
thousands)
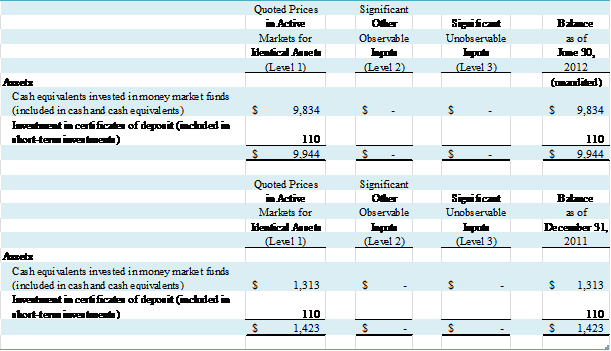
The fair values of the Company’s cash equivalents invested
in money market funds are determined through market, observable and
corroborated sources.
NOTE
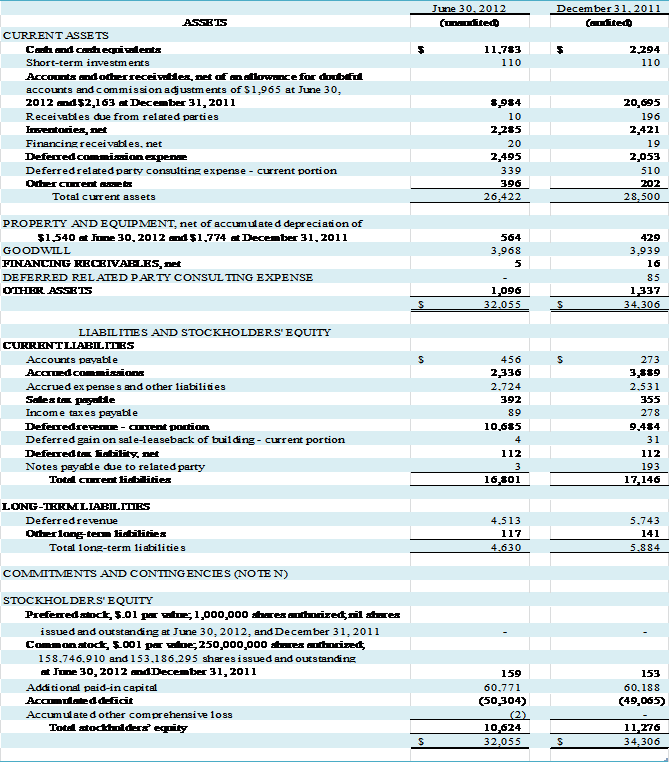
G – ACCOUNTS AND OTHER RECEIVABLES, NET
The following table presents information regarding the
Company’s accounts and other receivables as of June 30, 2012 and December 31,
2011:
(in
thousands)

Trade receivables include amounts due for shipped products
and services rendered. Amounts currently
due under the GEHC Agreement are subject to adjustment in subsequent periods
should the underlying sales order amount, upon which the receivable is based,
change.
Allowance for doubtful accounts and commission adjustments
include estimated losses resulting from the inability of our customers to make
required payments, and adjustments arising from subsequent changes in sales
order amounts that may reduce the amount the Company will ultimately receive
under the GEHC Agreement. Due from
employees primarily reflects commission advances made to sales personnel.
Inventories, net of reserves, consist of the
following: (in thousands)

At June 30, 2012 and December
31, 2011, the Company had reserves for excess and obsolete inventory of $546,000
and $606,000, respectively.
NOTE I– BUSINESS COMBINATION
On September 2, 2011,
Vasomedical Global successfully completed the purchase of all the outstanding
capital stock of privately-held Fast Growth Enterprises Limited (“FGE”), a
British Virgin Islands company that owns subsidiaries which own and control
Life Enhancement Technology Ltd (“LET”) and Biox
Instruments Co. Ltd. (“Biox”), respectively, as per
the stock purchase agreement signed on August 19, 2011. The consideration of
this acquisition includes a cash payment of $1 million as well as the issuance
of 5 million restricted shares of the Company’s common stock, up to 2.4 million
shares of common stock contingently issuable upon the achievement of certain
operating performance targets, and warrants covering 1.5 million shares of
common stock. The Company is completing
its evaluation of FGE’s calendar year 2011 results and believes it is likely
that the targets have been met and the shares will be issued.
LET, based in Foshan, Guangdong, China, is Vasomedical’s
supplier for its proprietary Enhanced External Counterpulsation
(EECP®) systems, including certain Lumenair
systems and all AngioNew® systems. Biox, a developer and manufacturer of ambulatory monitoring
devices, is located in Wuxi, Jiangsu, China, and is Vasomedical’s
supplier of the BIOX series ECG Holter recorder and
analysis software as well as ambulatory blood pressure monitoring systems.
Vasomedical has obtained FDA clearance to market these products in the United
States. The acquisition of LET provides
Vasomedical with consolidated technical and manufacturing capability in its
EECP business which has significantly increased gross margins and will enable
the Company to meet anticipated increasing demand for its EECP systems. The acquisition of Biox
greatly enhances Vasomedical's distribution network,
technology and product portfolio, and with combined market and sales efforts of
the two companies, has improved performance and profitability of Vasomedical's equipment segment.
The operating results of
FGE are included in the accompanying Consolidated Condensed Statements of
Operations and Comprehensive Income (Loss) and Cash Flows for the six and three
months ended June 30, 2012. The
Consolidated Condensed Balance Sheet as of June 30, 2012 reflects the acquisition
of FGE, effective September 2, 2011. The
acquisition date fair value of the total consideration transferred was $3.979
million, which consisted of the following:
(in
thousands)

In accordance with Accounting
Standards Codification ("ASC") 805, Business Combinations ("ASC
805"), the total purchase consideration is allocated to the net tangible
and identifiable intangible assets acquired and liabilities assumed based on
their estimated fair values as of September 2, 2011 (the acquisition
date). The purchase price was allocated
based on the information currently available, and may be adjusted after
obtaining more information regarding, among other things, asset valuations,
liabilities assumed, and revisions of preliminary estimates. The following table summarizes the estimated
fair values of the assets acquired and liabilities assumed at the acquisition
date:
(in thousands)

The goodwill is attributable to
the synergies expected to arise after the Company’s acquisition of FGE as well
as FGE’s projected growth and profitability.
The goodwill is not expected to be deductible for tax purposes.
After elimination of
intercompany transactions which reduce the income from FGE, the amounts of
revenue and net loss of FGE included in the Company’s Consolidated Condensed
Statement of Operations and Comprehensive Income (Loss) for the six months
ended June 30, 2012 was $910,000 and $24,000, respectively, and $332,000 and
$110,000, respectively, for the three months ended June 30, 2012. The effect of FGE’s loss on earnings per
share for the six and three months ended June 30, 2012 was $0.00.
The following supplemental pro
forma information presents the financial results as if the acquisition of FGE
had occurred June 1, 2009 (amounts in thousands, except per share amounts):
(in thousands
except per share data)
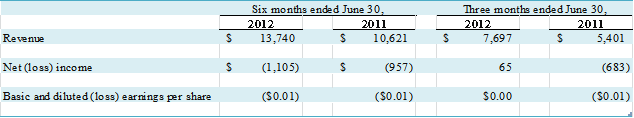
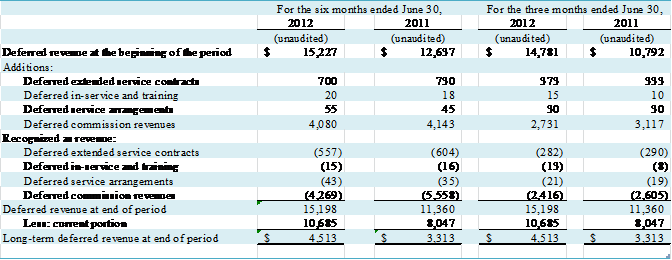
The changes in the Company’s deferred revenues are as
follows:
(in thousands)
NOTE L – RELATED-PARTY TRANSACTIONS
On June 21, 2007, we entered
into a Securities Purchase Agreement with Kerns Manufacturing Corp.
(“Kerns”). Pursuant to this agreement, a
five-year warrant to purchase 4,285,714 shares of our common stock at an
initial exercise price of $0.08 per share was issued to Kerns. In March 2012, Kerns exercised its warrant and
purchased 4,285,714 shares of common stock.
Concurrently with our entry into the Securities Purchase Agreement, we
also entered into a Distribution Agreement and a Supplier Agreement with Living
Data Technology Corporation (“Living Data”), an affiliate of Kerns. Pursuant to the Distribution Agreement, as
amended, we became the exclusive worldwide distributor of the AngioNew EECP® systems manufactured through
Living Data. The Distribution Agreement had an initial term extending through
May 31, 2012. Subsequent to August 31,
2011 the Company acquired Life Enhancement Technology (LET) (see Note I), the
manufacturer of the AngioNew EECP® system. Consequently, the Distribution Agreement is
no longer effective, and the Company wrote-off the remaining unamortized
balance of Deferred Distributor Costs during the seven months ended December
31, 2011.
On February 28, 2011, David Lieberman and Edgar Rios
were appointed by the Board of Directors as directors of the Company. Mr. Lieberman, a practicing attorney in the
State of New York, was appointed to serve as the Vice Chairman of the
Board. He is currently a senior partner at the law firm of Beckman,
Lieberman & Barandes, LLP,
which firm performs certain legal services for the Company. Fees of approximately $82,000 and $149,000 were
billed by the firm through the three and six months ended June 30, 2012,
respectively, at which date no amounts were outstanding.
Mr. Rios is President of Edgary
Consultants, LLC, and was appointed a director in conjunction with the
Company’s consulting agreement (the “Agreement”) with Edgary
Consultants, LLC (“Consultant”). The Agreement
commenced on March 1, 2011 and runs for a two year term. The
Agreement provides for the engagement of Consultant to assist the Company in
seeking broader reimbursement coverage of EECP® therapy. More
specifically, Consultant will be assisting the Company in the following areas:
1. Engaging
the adoption of EECP® therapy as a first line option for FDA cleared
indications as it relates to CCS Class III/IV angina with a major commercial
healthcare third-party payer.
2. Engaging
a major commercial healthcare payer to formally collaborate and co-sponsor a
study with Vasomedical for the efficacy, efficiency and/or cost effectiveness
of the EECP® therapy for NYHA Class II/III heart failure.
3. Engaging
final approval from the Centers for Medicare and Medicaid Services (“CMS”) of
EECP® therapy as a first line treatment for CCS Class III/IV angina.
4. Engaging
final approval from CMS to extend coverage and provide for the reimbursement of
EECP® therapy for CCS Class II angina; and
5. Engaging
final approval from CMS to extend coverage and provide for the reimbursement of
EECP® therapy for NYHA Class II/III heart failure.
In consideration for the services to be provided by
Consultant under the Agreement, the Company has agreed to issue to Consultant
or its designees, approximately 10% of the outstanding capital stock of
the Company, of which the substantial portion (in excess of 82%) is performance
based as referenced above. In conjunction with the Agreement, 3,000,000 shares
of restricted common stock valued at $1,020,000 were issued in March 2011. In connection with the Agreement, Mr.
Lieberman received 600,000 of these restricted shares. The Company has recorded the fair value of
the shares issued to Consultant as a prepaid expense and is amortizing the cost
ratably over the two year agreement. The
unamortized value is reported as Deferred Related Party Consulting Expense in our
accompanying consolidated condensed balance sheets as of June 30, 2012 and December
31, 2011.
During the six months ended June 30, 2012, a director
performed consulting services for the Company aggregating approximately $10,000.
Through the Company’s acquisition of FGE in September 2011,
it assumed the liability for $288,000 in unsecured notes payable to the
President of LET and his spouse, of which $95,000 was repaid in December 2011,
and $190,000, bearing interest at 6% per annum, was paid in March 2012. In addition, receivables due from FGE
management aggregating $159,000 were collected during the six months ended June
30, 2012.
NOTE M –
STOCKHOLDERS’ EQUITY
Common Stock
On June 17, 2010 the Board of Directors approved the 2010
Stock Plan (the “2010 Plan”) for officers, directors, employees and consultants
of the Company. The stock issuable under
the 2010 Plan shall be shares of the Company’s authorized but unissued or
reacquired common stock. The maximum
number of shares of common stock which may be issued under the 2010 Plan is 5,000,000
shares.
The 2010 Plan is comprised of two separate equity programs,
the Options Grant Program, under which eligible persons may be granted options
to purchase shares of common stock, and the Stock Issuance Program, under which
eligible persons may be issued shares of common stock directly, either through
the immediate purchase of such shares or as compensation for services rendered
to the Company.
The 2010 Plan provides that the Board of Directors, or a
committee of the Board of Directors, will administer it with full authority to
determine the identity of the recipients of the options or shares and the
number of options or shares. Options
granted under the 2010 Plan may be either incentive stock options or
non-qualified stock options. The option price
shall be 100% of the fair market value of the common stock on the date of the
grant ( or in the case of incentive stock options granted to any individual
stockholder possessing more than 10% of the total combined voting power of all
voting stock of the Company, 110% of such fair market value). The term of any option may be fixed by the Board
of Directors, or its authorized committee, but in no event shall it exceed five
years from the date of grant. Options
are exercisable upon payment in full of the exercise price, either in cash or
in common stock valued at fair market value on the date of exercise of the
option.
As of June 30, 2012, 3,790,000 restricted shares of common
stock were granted under the 2010 Plan to non-officer employees and consultants
of the Company. As of June 30, 2012, 755,000
shares have been forfeited. In March 2012,
500,000 restricted shares of common stock were granted under the 2010 Plan to
an officer, of which 250,000 vested immediately with the remainder vesting over
a one year period. In June 2012,
2,190,000 additional shares of restricted common stock were granted to
non-officer employees in conjunction with the extension of the GEHC Agreement,
vesting at various times through July 1, 2013.
In July 2012, 500,000 shares of restricted common stock were granted to
non-officer employees, of which 250,000 vest within one year and the remainder
one year thereafter.
No options were issued under the 2010 Plan during the six months
ended June 30, 2012 and 2011.
In September 2011, the Company issued 5,000,000 shares of
restricted common stock and a two year common stock purchase warrant for
1,500,000 shares at an exercise price of $0.50 per share as partial
consideration for the acquisition of FGE.
In addition, up to 2,400,000 shares of common stock are contingently
issuable should FGE attain certain operating targets for the twelve months
ending December 31, 2011. The Company is
completing its evaluation of FGE’s calendar year 2011 results and believes it
is likely that the targets have been met and the shares will be issued. The aggregate value of the aforementioned
noncash consideration relative to the FGE acquisition was $2,979,000.
Preferred Stock
On June 24, 2010, the Company filed a Certificate of
Designations of Preferences and Rights of Series E Convertible Preferred Stock
(“Certificate of Designations”), as authorized by the Board of Directors,
designating 350,000 shares of its 1,000,000 shares of preferred stock as Series
E Convertible Preferred Stock (“Series E Preferred”). The conversion rights of
the Series E Preferred are that each share will be convertible at any time on
or after January 1, 2011, at the holder’s option into 100 shares of common
stock (an exercise price of $.16 per share of common stock, the “Conversion
Price”), subject to anti-dilution adjustment as set forth below. Each share of outstanding Series E Preferred
Stock shall automatically be converted into shares of common stock on or after
July 1, 2011, at the then effective applicable conversion ratio, if, at any
time following the Issuance Date, the price of the common stock for any 30
consecutive trading days equals or exceeds three times the Conversion Price and
the average daily trading volume for the Company’s common stock for the 30
consecutive trading days exceeds 250,000 shares.
Pursuant to its conversion terms, the Series E Preferred
was deemed automatically converted to common stock effective July 1, 2011. As of June 30, 2012, 30,668,500 shares of
common stock had been issued for 306,685 shares of Series E Preferred.
For the three and six months ended June 30, 2011, the
Company sold 0 and 9,375 shares of Series E Preferred aggregating $150,000, and
recorded dividends totaling $151,000 and $279,000, respectively. Included in such dividends is the recognition
of the value of the embedded beneficial conversion feature of the Series E
Preferred, which reflects the difference between the conversion price and the
market price at time of investment. The amounts included in the dividends reported
attributable to this beneficial conversion feature are $91,000 and $156,000 for
the three and six months ended June 30, 2011, respectively. These are noncash dividends requiring no
payment and ceased on conversion of the Series E Preferred to common stock.
NOTE N – COMMITMENTS
AND CONTINGENCIES
Sales representation agreement
In June 2012, the Company concluded an
amendment of the GEHC Agreement with GE Healthcare, originally signed on May
19, 2010. The amendment, effective July
1, 2012, extends the initial term of three years commencing July 1, 2010 to
five years through June 30, 2015, subject to earlier termination under certain
circumstances. These circumstances
include not materially achieving certain sales goals, not maintaining a minimum
number of sales representatives, and various legal and GEHC policy
requirements. Under the terms of the
agreement, the Company is required to lease dedicated computer equipment from GEHC
for connectivity to their network.
In conjunction with the extension of the GEHC
Agreement, the Company granted VasoHealthcare employees both stock and
cash-based performance incentives for the ensuing year. The incentives provide for cash payments of
up to $2.2 million and 2.2 million shares of restricted common stock grants and
vest at various times through July 1, 2013.
A condition of the incentives is that the employees remain continuously
employed through the vesting dates.
NOTE O - RECENTLY ISSUED ACCOUNTING
PRONOUNCEMENTS
Other Comprehensive Income: Presentation of
Comprehensive Income
In June 2011, new guidance was issued that amends the
current comprehensive income guidance. The new guidance allows the option to
present the total of comprehensive income, the components of net income, and
the components of other comprehensive income either in a single or continuous
statement of comprehensive income or in two separate but consecutive
statements. The amendments in this update do not change the items that must be
reported in other comprehensive income or when an item of other comprehensive
income must be reclassified to net income. The new guidance is to be applied
retrospectively and is effective for fiscal years, and interim periods,
beginning after December 15, 2011, with early adoption permitted. The adoption of this guidance did not have a
material impact on the Company’s consolidated condensed financial statements.
In December 2011, the FASB issued authoritative guidance to
defer the effective date for those aspects of the guidance relating to the
presentation of reclassification adjustments out of accumulated other
comprehensive income. The adoption of this new guidance will not have an impact
on the Company’s consolidated financial position, results of operations or cash
flows as it only requires a change in the format of the current presentation of
other comprehensive income.
Except for
historical information contained in this report, the matters discussed are
forward-looking statements that involve risks and uncertainties. When used in
this report, words such as “anticipates”, “believes”, “could”, “estimates”,
“expects”, “may”, “plans”, “potential” and “intends” and similar expressions,
as they relate to the Company or its management, identify forward-looking
statements. Such forward-looking statements are based on the beliefs of the
Company’s management, as well as assumptions made by and information currently
available to the Company’s management. Among the factors that could cause
actual results to differ materially are the following: the effect of business and economic conditions; the effect of the dramatic changes taking place in the
healthcare environment; the impact of competitive procedures and products and
their pricing; medical insurance reimbursement policies; unexpected
manufacturing or supplier problems; unforeseen difficulties and delays in the
conduct of clinical trials and other product development programs; the actions
of regulatory authorities and third-party payers in the United States and
overseas; uncertainties about the acceptance of a novel therapeutic modality by
the medical community; continuation of the GEHC Agreement and the risk factors
reported from time to time in the Company’s SEC reports, including its recent
transition report on Form 10-K. The
Company undertakes no obligation to update forward-looking statements as a
result of future events or developments.
General Overview
Vasomedical, Inc. was incorporated in Delaware in July
1987. Unless the context requires
otherwise, all references to “we”, “our”, “us”, “Company”, “registrant”,
“Vasomedical” or “management” refer to Vasomedical, Inc. and its subsidiaries. Until 2010, we were primarily engaged in
designing, manufacturing, marketing and supporting EECP® Enhanced
External Counterpulsation systems, based on our
proprietary technology, to physicians and hospitals throughout the United
States and in select international markets.
In May 2010, the Company, through its wholly-owned
subsidiary Vaso Diagnostics, Inc. d/b/a VasoHealthcare, expanded into the sales
representation business via its agreement with GE Healthcare (“GEHC”), the
healthcare business unit of General Electric Company (NYSE: GE), to be GEHC’s
exclusive sales representative for the sale of select GEHC diagnostic imaging
products in specific market segments in the 48 contiguous states of the United
States and the District of Columbia. In June 2012, the Company
entered into an amendment, effective July 1, 2012, of the sales representative
agreement (“GEHC Agreement”) extending the initial term of three years
commencing July 1, 2010 to five years through June 30, 2015, subject to earlier
termination under certain circumstances.
In September 2011, the Company acquired Fast Growth
Enterprises Limited (FGE), a British Virgin Islands company, which, through its
subsidiaries, owns and controls two Chinese operating companies - Life
Enhancement Technology Ltd. and Biox Instruments Co.
Ltd., respectively - to expand its technical and manufacturing capabilities and
to enhance its distribution network, technology, and product portfolio. Also in September 2011, the Company
restructured to further align its business management structure and long-term
growth strategy, and now operates through three wholly-owned subsidiaries. Vaso Diagnostics
d/b/a VasoHealthcare continues as the operating subsidiary for the sales
representation of GE diagnostic imaging products; Vasomedical Global Corp.
operates the Company’s newly-acquired Chinese companies; and Vasomedical
Solutions, Inc. was formed to manage and coordinate our EECP® therapy
business as well as other medical equipment operations.
We now report the operations of Vasomedical Global Corp.
and Vasomedical Solutions, Inc. under our Equipment reportable segment. VasoHealthcare activities are included under
our Sales Representation reportable segment (see Note C).
The Company will seek to improve
profitability through our recent accretive acquisition of the two Chinese
medical device companies and by expanding our U.S. market product
portfolio. In addition, the Company
plans to actively pursue other accretive acquisitions in the international
market and is in preliminary discussions to secure a credit facility for up to
$25 million to be utilized for this purpose.
Critical Accounting Policies and Estimates
Our discussion and analysis of
our financial condition and results of operations are based upon the
accompanying unaudited consolidated condensed financial statements, which have
been prepared in accordance with accounting principles generally accepted in
the United States (“U.S. GAAP”). The preparation of financial statements in
conformity with U.S. GAAP requires management to make judgments, estimates and
assumptions that affect the reported amounts of assets, liabilities, revenue,
expenses, and the related disclosures at the date of the financial statements
and during the reporting period. Although these estimates are based on our
knowledge of current events, our actual amounts and results could differ from
those estimates. The estimates made are based on historical factors, current
circumstances, and the experience and judgment of our management, who
continually evaluate the judgments, estimates and assumptions and may employ outside
experts to assist in the evaluations.
Certain of our accounting
policies are deemed “critical”, as they are both most important to the
financial statement presentation and require management’s most difficult,
subjective or complex judgments as a result of the need to make estimates about
the effect of matters that are inherently uncertain. For a discussion of our
critical accounting policies, see “Management’s Discussion and Analysis of
Financial Condition and Results of Operations” in our Transition Report on Form
10-K for the seven months ended December 31, 2011.
On June 15, 2011, the Board of Directors approved a change
of the Company’s fiscal year end from May 31st to December 31st. As a result of this change, the Company filed
a Transition Report on Form 10-K for the seven-month transition period ended
December 31, 2011. Going forward,
references to our 2012 and 2011 fiscal years herein mean the fiscal years ended
December 31, 2012 and December 31, 2011, respectively.
New Accounting Pronouncements - Adoption of New Standards
Other Comprehensive Income: Presentation of
Comprehensive Income
In June 2011, new guidance was issued that amends the
current comprehensive income guidance. The new guidance allows the option to
present the total of comprehensive income, the components of net income, and
the components of other comprehensive income either in a single or continuous
statement of comprehensive income or in two separate but consecutive
statements. The amendments in this update do not change the items that must be
reported in other comprehensive income or when an item of other comprehensive
income must be reclassified to net income. The new guidance is to be applied
retrospectively and is effective for fiscal years, and interim periods, beginning
after December 15, 2011, with early adoption permitted. The adoption of this guidance did not have a
material impact on the Company’s consolidated financial statements.
In December 2011, the FASB issued authoritative guidance to
defer the effective date for those aspects of the guidance relating to the
presentation of reclassification adjustments out of accumulated other
comprehensive income. The adoption of this new guidance will not have an impact
on the Company’s consolidated financial position, results of operations or cash
flows as it only requires a change in the format of the current presentation of
other comprehensive income.
Consolidated Results of
Operations
Three Months Ended June 30, 2012 and June 30,
2011
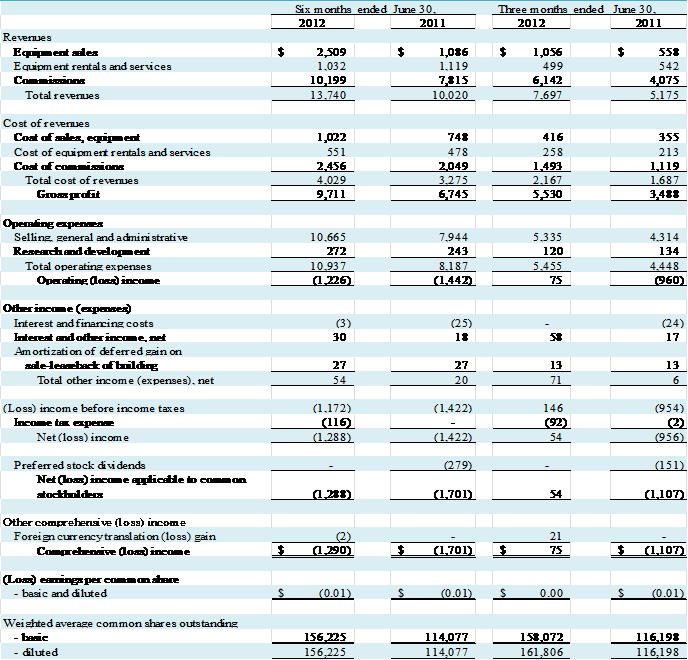
Total revenue for the three months ended June 30, 2012 and June
30, 2011, was $7,697,000 and $5,175,000, respectively, an increase of $2,522,000,
or 49%. Net income for the three months ended June 30, 2012 was $54,000
compared to a net loss of $956,000 for the three months ended June 30,
2011. We reported net income applicable
to common stockholders of $54,000 for the second quarter of fiscal year 2012
compared to a net loss applicable to common stockholders of $1,107,000 for the second
quarter of fiscal year 2011. The change from net loss to net income applicable
to common stockholders was primarily attributable to a $2,042,000 increase in
gross profit, partially offset by a $1,021,000 increase in selling, general and
administrative (“SG&A”) costs (of which $625,000 was attributable to
VasoHealthcare). Our total net earnings
(loss) was $0.00 and ($0.01) per basic and diluted common share for the three
months ended June 30, 2012 and 2011, respectively.
Revenues
Revenue in our Equipment segment
increased by $455,000, or 41%, to $1,555,000 for the three-month period ended June
30, 2012 from $1,100,000 for the same period of the prior year. Equipment segment revenue from equipment
sales increased by $498,000, or 89%, to $1,056,000 for the three-month period
ended June 30, 2012 as compared
to $558,000 for the same period in the prior year. The increase in equipment
sales is due primarily to an increase in the number of EECP® units
shipped as well as an increase in the sales price per EECP® unit,
partially offset by a decrease in sales of other medical equipment, and by the
inclusion of $332,000 in equipment sales generated by our Chinese subsidiaries
acquired in September, 2011.
Current demand for EECP®
systems will likely remain soft until there is greater clinical acceptance for
the use of EECP® therapy in treating patients with angina or angina
equivalent symptoms who meet the current reimbursement guidelines, or a
favorable change in current reimbursement policies by CMS or third party payers
to consider EECP therapy as a first-line treatment option for angina or cover
some or all Class II & III heart failure patients. Patients with angina or
angina equivalent symptoms eligible for reimbursement under current policies
include many with serious comorbidities, such as heart failure, diabetes,
peripheral vascular disease and/or others.
As described in Note L, we are pursuing initiatives to expand
reimbursement that we expect should ultimately increase overall market demand
for our EECP® systems.
Equipment segment revenue from
equipment rental and services decreased 8% to $499,000 in the second quarter of
2012 from $542,000 in the second quarter of 2011. Revenue from equipment rental
and services represented 32% and 49% of total Equipment segment revenue in the second
quarters of fiscal 2012 and fiscal 2011, respectively. The decrease in revenue generated from
equipment rentals and services is due primarily to decreased accessory part
revenue.
Commission revenues in the Sales
Representation segment were $6,142,000 in the second quarter of 2012, as
compared to $4,075,000 in the second quarter of 2011, an increase of 51%. The increase in commission revenue in the second
quarter of 2012 is due to increased volume of equipment delivered as well as
higher blended commission rates. The Company
recognizes revenue when the underlying equipment has been accepted at the
customer site in accordance with the specific terms of the sales
agreement. Consequently, amounts
billable under the agreement with GE Healthcare prior to customer acceptance of
the equipment are recorded as deferred revenue in the Consolidated Condensed
Balance Sheet. Due to the nature of our
commission structure under the GEHC Agreement, wherein the Company earns
progressively higher commission rates retroactively as calendar year targets
are met, revenues earned in the first and fourth quarters typically reflect a
higher blended commission rate than revenues earned in the second and third
quarters. As of June 30, 2012, $13,896,000
in deferred commission revenue was recorded in the Company’s consolidated
condensed balance sheet, of which $4,068,000 is long-term. At June 30, 2011, $10,275,000 in deferred
commission revenue was recorded in the Company’s consolidated condensed balance
sheet, of which $2,980,000 was long-term.
Gross Profit
The Company had a gross profit
of $5,530,000 in the second quarter of 2012 compared to $3,488,000 in the second
quarter of the prior year, an increase of 59%.
Equipment segment gross profit increased to $881,000, or 57% of
Equipment segment revenues, for the second quarter of 2012 compared to $532,000
or 48% of Equipment segment revenues, for the same quarter of 2011. Equipment segment gross profit was favorably
impacted both in absolute dollars and gross profit percentage by the $332,000
in sales of other medical equipment sold through our Chinese subsidiaries in
the second quarter of 2012. In addition,
gross profit on EECP® equipment improved due to lower costs arising
from the acquisition of LET, our primary supplier of certain EECP®
systems. Gross profit in the Equipment
segment is dependent on a number of factors, particularly the mix of new and
refurbished EECP® systems and the mix of models sold, their
respective average selling prices, the mix of EECP® units sold,
rented or placed during the period, the ongoing costs of servicing EECP®
systems, and certain fixed period costs, including facilities, payroll and
insurance.
Sales Representation segment
gross profit was $4,649,000, or 76%, for the three months ended June 30, 2012
as compared to $2,956,000, or 73%, for the three months ended June 30, 2011. The increase was due both to higher sales
volume and higher blended commission rates in the second quarter of 2012. Cost of commissions of $1,493,000 and $1,119,000,
for the three months ended June 30, 2012 and 2011, respectively, reflects
commission expense associated with recognized commission revenues. Commission expense associated with deferred
revenue is recorded as deferred commission expense until the related commission
revenue is earned.
Operating Income (Loss)
Operating income was $75,000 for the three months ended June
30, 2012 as compared to an operating loss of $960,000 for the three months
ended June 30, 2011, an improvement of $1,035,000. The improvement in operating income was primarily
attributable to improved operating performance in the Sales Representation
segment, where operating income was $681,000 for the second quarter of 2012
compared to an operating loss of $386,000 in the same quarter of the prior
year, partially offset by higher corporate expenses.
Selling, general and
administrative (“SG&A”) expenses for the second quarter of 2012 and 2011
were $5,335,000, or 69% of revenues, and $4,314,000, or 83% of revenues,
respectively, reflecting an increase of $1,021,000 or approximately 24%. The
increase in SG&A expenditures in the second quarter of 2012 resulted
primarily from increased sales and marketing costs in both segments, including
SG&A costs of the recently acquired Chinese entities, and higher corporate compensation
costs.
During the second quarter of 2012, the Company recorded a reduction
in its provision for doubtful accounts and commission adjustments of $9,000 as
compared to the second quarter of 2011 when the Company recorded a reduction in
its provision for doubtful accounts and commission adjustments of $3,000.
Research
and development (“R&D”) expenses of $120,000, or 2% of revenues, for the second
quarter of 2012 decreased by $14,000, or 10%, from $134,000, or 3% of revenues,
for the second quarter of 2011. The decrease is primarily attributable to a decrease
in clinical research expenses.
Interest and Financing Costs
No interest and financing costs were
incurred in the second quarter of 2012, as compared to $24,000 incurred in the
second quarter of 2011. Interest and
financing costs for the second quarter of 2011 consisted of interest on a
short-term note to finance the Company’s insurance premiums.
Interest and Other Income, Net
Interest and other income
for the second quarters of 2012 and 2011, was $58,000 and $17,000,
respectively. The increase of $41,000 in the second quarter of 2012 is due
primarily to value-added tax refunds recognized by our Chinese subsidiaries, as
well as to higher interest earned on the Company’s cash balances.
Amortization of Deferred Gain on Sale-leaseback of Building
The amortization of deferred
gain on sale-leaseback of building for the second quarters of 2012 and
2011 was $13,000. The gain resulted
from the Company’s sale-leaseback of its facility.
Income Tax Expense, Net
During
the second quarter of year 2012 we recorded a provision for income taxes of $84,000
as compared to a provision of $2,000 for the second quarter of 2011. The increase arose primarily from foreign tax
liabilities associated with the acquisition of FGE.
Six Months Ended June 30, 2012 and June 30,
2011
Total revenue for the six months ended June 30, 2012 and
June 30, 2011, was $13,740,000 and $10,020,000, respectively, an increase of $3,720,000,
or 37%. Net loss for the six months ended June 30, 2012 was $1,288,000 compared
to a net loss of $1,422,000 for the six months ended June 30, 2011. We reported net loss applicable to common
stockholders of $1,288,000 for the first two quarters of 2012 compared to a net
loss applicable to common stockholders of $1,701,000 for the first two quarters
of 2011. The decrease in net loss applicable to common stockholders was
primarily attributable to a $2,966,000 increase in gross profit and $279,000
reduction in preferred stock dividends, partially offset by a $2,721,000
increase in selling, general and administrative (“SG&A”) costs (of which $1,674,000
was attributable to VasoHealthcare). Our
total net loss was $0.01 per basic and diluted common share for the six months
ended June 30, 2012 and 2011.
Revenues
Revenue in our Equipment segment
increased by $1,336,000, or 61%, to $3,541,000 for the six-month period ended
June 30, 2012 from $2,205,000 for the same period of the prior year. Equipment segment revenue from equipment
sales increased by $1,423,000, or 131%, to $2,509,000 for the six-month period
ended June 30, 2012 as compared
to $1,086,000 for the same period in the prior year. The increase in equipment
sales is due primarily to an increase in the number of EECP® units
shipped as well as an increase in the sales price per EECP® unit,
partially offset by a decrease in sales of other medical equipment, and by the
inclusion of $910,000 in equipment sales generated by our Chinese subsidiaries
acquired in September, 2011.
Current demand for EECP®
systems will likely remain soft until there is greater clinical acceptance for
the use of EECP® therapy in treating patients with angina or angina
equivalent symptoms who meet the current reimbursement guidelines, or a
favorable change in current reimbursement policies by CMS or third party payers
to consider EECP therapy as a first-line treatment option for angina or cover
some or all Class II & III heart failure patients. Patients with angina or
angina equivalent symptoms eligible for reimbursement under current policies
include many with serious comorbidities, such as heart failure, diabetes,
peripheral vascular disease and/or others.
As described in Note L, we are pursuing initiatives to expand reimbursement
that we expect should ultimately increase overall market demand for our EECP®
systems.
Equipment segment revenue from
equipment rental and services decreased 8% to $1,032,000 in the first six
months of 2012 from $1,119,000 in the first six months of 2011. Revenue from
equipment rental and services represented 29% and 51% of total Equipment
segment revenue in the first six months of 2012 and 2011, respectively. The decrease in revenue generated from
equipment rentals and services is due primarily to decreased service
revenue.
Commission revenues in the Sales
Representation segment were $10,199,000 in the first six months of 2012, as
compared to $7,815,000 in the first six months of 2011, an increase of 30%. The increase in commission revenue in the
first six months of 2012 is due to increased volume of equipment delivered as
well as higher blended commission rates.
The Company recognizes revenue when the underlying equipment has been
accepted at the customer site in accordance with the specific terms of the
sales agreement. Consequently, amounts
billable under the agreement with GE Healthcare prior to customer acceptance of
the equipment are recorded as deferred revenue in the Consolidated Condensed
Balance Sheet. Due to the nature of our
commission structure under the GEHC Agreement, wherein the Company earns
progressively higher commission rates retroactively as calendar year targets
are met, revenues earned in the first and fourth fiscal quarters typically
reflect a higher blended commission rate than revenues earned in the second and
third fiscal quarters. As of June 30,
2012, $13,896,000 in deferred commission revenue was recorded in the Company’s
consolidated condensed balance sheet, of which $4,068,000 is long-term. At June 30, 2011, $10,275,000 in deferred
commission revenue was recorded in the Company’s consolidated condensed balance
sheet, of which $2,980,000 was long-term.
Gross Profit
The Company had a gross profit
of $9,711,000 in the first six months of 2012 compared to $6,745,000 in the
first six months of the prior year, an increase of 44%. Equipment segment gross profit increased to $1,968,000,
or 56% of Equipment segment revenues, for the first six months of 2012 compared
to $979,000 or 44% of Equipment segment revenues, for the first six months of
2011. Equipment segment gross profit was
favorably impacted both in absolute dollars and gross profit percentage by the
$910,000 in sales of other medical equipment sold through our Chinese
subsidiaries in the first six months of 2012.
In addition, gross profit on EECP® equipment improved due to
lower costs arising from the acquisition of LET, our primary supplier of
certain EECP® systems. Gross
profit in the Equipment segment is dependent on a number of factors,
particularly the mix of new and refurbished EECP® systems and the
mix of models sold, their respective average selling prices, the mix of EECP®
units sold, rented or placed during the period, the ongoing costs of servicing
EECP® systems, and certain fixed period costs, including facilities,
payroll and insurance.
Sales Representation segment
gross profit was $7,743,000 for the six months ended June 30, 2012 as compared
to $5,766,000 for the six months ended June 30, 2011. Cost of commissions of $2,456,000 and $2,048,000,
for the six months ended June 30, 2012 and 2011, respectively, reflects
commission expense associated with recognized commission revenues. Commission expense associated with deferred
revenue is recorded as deferred commission expense until the related commission
revenue is earned.
Operating Loss
Operating loss decreased by $216,000, or 15%, to $1,226,000
for the six months ended June 30, 2012 as compared to an operating loss of $1,442,000
for the six months ended June 30, 2011.
The decrease in operating loss was primarily attributable to lower
operating losses in the Sales Representation segment of $303,000 and Equipment
segment of $388,000, both arising from higher revenues and gross profit,
partially offset by higher corporate expenses of $475,000.
Selling, general and
administrative (“SG&A”) expenses for the first six months of 2012 and 2011
were $10,665,000, or 78% of revenues, and $7,944,000, or 79% of revenues,
respectively, reflecting an increase of $2,721,000 or approximately 34%. The
increase in SG&A expenditures in the first six months of 2012 resulted
primarily from increased sales and marketing costs in both segments, including
SG&A costs of the recently acquired Chinese entities, and higher corporate
compensation costs.
During the first six months of 2012, the Company recorded a
reduction in its provision for doubtful accounts and commission adjustments of
$3,000 as compared to the first six months of 2011 when the Company increased
its provision for doubtful accounts and commission adjustments by $9,000.
Research
and development (“R&D”) expenses of $272,000, or 2% of revenues, for the
first six months of 2012 increased by $29,000, or 12%, from $243,000, or 2% of
revenues, for the first six months of 2011. The increase is primarily
attributable to an increase in regulatory costs.
Interest and Financing Costs
Interest and financing costs for
the first six months of 2012 and 2011 were $3,000 and $25,000,
respectively. Interest and financing
costs for the first six months of 2012 consisted primarily of interest on the notes
payable to a related party in one of the China subsidiaries. Interest and financing costs for the first six
months of 2011 consisted of interest on a short-term note to finance the
Company’s insurance premiums.
Interest and Other Income, Net
Interest and other income
for the first six months of 2012 and 2011, was $30,000 and $18,000,
respectively. The increase of $12,000 in the first six months of 2012 is due
primarily to value-added tax refunds recognized by our Chinese subsidiaries, as
well as to higher interest earned on the Company’s cash balances.
Amortization of Deferred Gain on Sale-leaseback of Building
The amortization of deferred
gain on sale-leaseback of building for the first six months of 2012 and
2011 was $27,000. The gain resulted from
the Company’s sale-leaseback of its facility.
Income Tax Expense, Net
During
the first six months of 2012 we recorded a provision for income taxes of $108,000
as compared to the first six months of 2011 when no tax expense was recognized. The increase arose primarily from foreign tax
liabilities associated with the acquisition of FGE.
Liquidity and Capital Resources
Cash and Cash Flow
We have financed our operations
primarily from working capital, and, in the first six months of 2011, from the
issuance of the Company’s Series E Preferred Stock. At June 30, 2012, we had cash and cash equivalents of $11,783,000,
short-term investments of $110,000 and working capital of $9,621,000 compared
to cash and cash equivalents of $2,294,000, short-term investments of $110,000
and working capital of $11,354,000 at December 31, 2011.
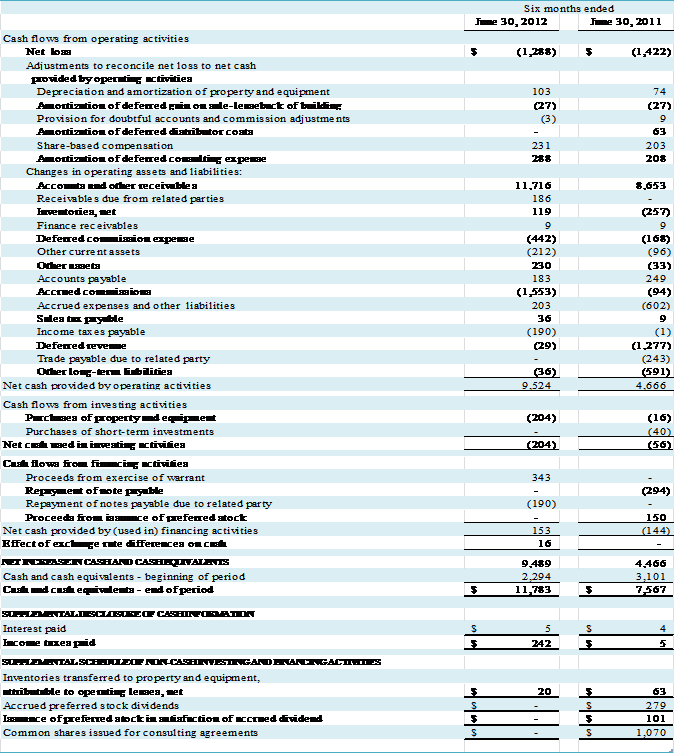
Cash provided by operating
activities was $9,524,000 during the first six months of 2012, which consisted
of a net loss after adjustments to reconcile net loss to net cash of $696,000,
offset by cash provided by operating assets and liabilities of $10,220,000. The
changes in the account balances primarily reflect a decrease in accounts and
other receivables of $11,716,000, offset by a decrease in accrued commissions
of $1,553,000 and an increase in deferred commission expense of $442,000. As noted above, under the GEHC Agreement the
Company earns progressively higher commission rates as calendar year targets
are met, which also has a significant impact on our cash flows. As we achieve
these targets the higher commission rates are retroactive to the beginning of
the calendar year, and therefore, the significantly higher commission billings
and recognized revenue generated in the fourth quarter of 2011 resulted in
significant cash inflows in the first quarter of 2012.
Cash used in investing activities during the six-month
period ended June 30, 2012 was $204,000
for the purchase of property and equipment.
Financing activities during the six-month
period ended June 30, 2012 provided
cash of $153,000, consisting of $343,000 provided through the exercise of
warrants, offset by $190,000 for the repayment of notes payable to a related
party.
Liquidity
The Company will seek to improve
profitability through our recent accretive acquisition of the two Chinese
medical device companies and by expanding our U.S. market product
portfolio. In addition, the Company
plans to actively pursue other accretive acquisitions in the international
market and is in preliminary discussions to secure a credit facility for up to
$25 million to be utilized for this purpose.
While we expect to generate
positive operating cash flows for 2012, the progressive nature of the
commission rates under the GEHC Agreement can cause related cash inflows to
vary widely during the year.
Evaluation of Disclosure Controls and Procedures
Disclosure
controls and procedures reporting as promulgated under the Exchange Act is
defined as controls and procedures that are designed to ensure that information
required to be disclosed by us in the reports that we file or submit under the
Exchange Act are recorded, processed, summarized and reported within the time
periods specified in the SEC rules and forms.
Disclosure controls and procedures include without limitation, controls
and procedures designed to ensure that information required to be disclosed by
us in the reports that we file or submit under the Exchange Act is accumulated
and communicated to our management, including our Chief Executive Officer
(“CEO”) and Chief Financial Officer (“CFO”), or persons performing similar
functions, as appropriate to allow timely decisions regarding required
disclosure.
Our CEO and our CFO have
evaluated the effectiveness of the design and operation of our disclosure
controls and procedures as of June 30, 2012 and have concluded that the
Company’s disclosure controls and procedures were not effective as of June 30,
2012 due to insufficient controls and management review over the recording of
certain transactions, and to lack of accounting personnel with appropriate
level of knowledge and experience in accounting principles generally accepted
in the United States of America and related accounting systems and closing
process at our China subsidiaries. The
Company intends to engage additional accounting personnel, including an
accounting controller, and strengthen its internal controls with regard to its
closing process, related disclosures, and the approval of certain transactions.
Changes in Internal Control Over Financial
Reporting
There
was no change in the Company’s internal control over financial reporting during
the Company’s last fiscal quarter that has materially affected, or is
reasonably likely to materially affect, the Company’s internal control over
financial reporting.
Exhibits
31 Certifications of the Chief Executive
Officer and the Chief Financial Officer pursuant to Rules 13a-14(a) as adopted
pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
32 Certifications of the Chief Executive
Officer and the Chief Financial Officer pursuant to 18 U.S.C. Section 1350 as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
In accordance with the requirements of the Exchange Act, the
Registrant caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
VASOMEDICAL,
INC.
By: /s/ Jun Ma
Jun Ma
President
and Chief Executive Officer
(Principal
Executive Officer)
/s/ Michael
J. Beecher .
Michael
J. Beecher
Chief
Financial Officer and Principal Accounting Officer
Date: August 14, 2012
![]()